Dimauro 2009 Biochim Biophys Acta
| DiMauro S, Rustin P (2009) A critical approach to the therapy of mitochondrial respiratory chain and oxidative phosphorylation diseases. Biochim Biophys Acta 1792:1159-67. https://doi.org/10.1016/j.bbadis.2008.10.015 |
DiMauro S, Rustin P (2009) Biochim Biophys Acta
Abstract: Taking advantage of a series of questions raised by an association of patients with mitochondrial disease, this review, after a brief overview of basic concepts of mitochondrial bioenergetics and genetics, discusses the pros and cons of a number of practical options in the field of mitochondrial therapy. This makes it clear that, in contrast to the spectacular progress in our understanding of the biochemical and molecular bases of the mitochondrial diseases defined restrictively as disorders due to defects in the mitochondrial respiratory chain, we are still extremely limited in our ability to treat these conditions. We finally discussed the emerging genetic-based strategies that show some promise, even if much work remains to be done.
• Bioblast editor: Gnaiger E
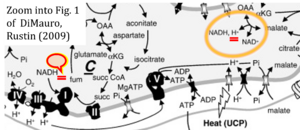
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 1 of DiMauro and Rustin (2009) shows explicitly reduction of NAD+ to NADH + H+ linked to oxidation of malate to oxaloacetate (OXA). Consistency in H+ balance then requires to indicate NADH + H+ as substrate(s) of Complex I.