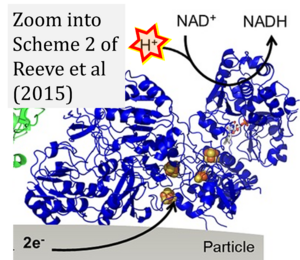
Reeve 2015 ChemCatChem
| Reeve HA, Lauterbach L, Lenz O, Vincent KA (2015) Enzyme-modified particles for selective biocatalytic hydrogenation by hydrogen-driven NADH recycling. ChemCatChem 7:3480-7. https://doi.org/10.1002/cctc.201500766 |
Reeve HA, Lauterbach L, Lenz O, Vincent KA (2015) ChemCatChem
Abstract: We describe a new approach to selective H2-driven hydrogenation that exploits a sequence of enzymes immobilised on carbon particles. We used a catalyst system that comprised alcohol dehydrogenase, hydrogenase and an NAD+ reductase on carbon black to demonstrate a greater than 98 % conversion of acetophenone to phenylethanol. Oxidation of H2 by the hydrogenase provides electrons through the carbon for NAD+ reduction to recycle the NADH cofactor required by the alcohol dehydrogenase. This biocatalytic system operates over the pH range 6-8 or in un-buffered water, and can function at low concentrations of the cofactor (10 μm NAD+) and at H2 partial pressures below 1 bar. Total turnover numbers >130 000 during acetophenone reduction indicate high enzyme stability, and the immobilised enzymes can be recovered by a simple centrifugation step and re-used several times. This offers a route to convenient, atom-efficient operation of NADH-dependent oxidoreductases for selective hydrogenation catalysis.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.