Difference between revisions of "Laner 2015 Abstract MiPschool Greenville 2015"
| (18 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{Abstract | {{Abstract | ||
|title= | |title=Comparative mitochondrial physiology: OXPHOS and ET capacity in permeabilized fibers of canine superathletes. | ||
|info=[[File:VG.jpg|150px|right|Laner V]] | |||
|authors=Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E | |authors=Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E | ||
|year=2015 | |year=2015 | ||
|event=MiPschool Greenville 2015 | |event=MiPschool Greenville 2015 | ||
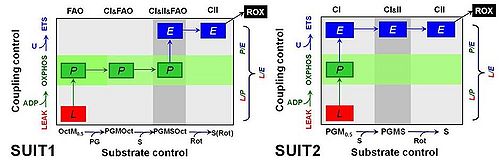
|abstract= | |abstract=Comparative mitochondrial physiology strongly relies on quantitative data sets for comparison of OXPHOS capacities and respiratory control patterns between species and tissues. Combination and interpretation of a wide variety of studies requires standardization of respiratory protocols, implementation of quality control criteria, and consistency of normalization. Previously, we described a reference method for the application of a cytochrome c threshold as exclusion criterion in mitochondrial OXPHOS analyses [1]. Alaskan sled dogs (N=6) were studied 72 to 120 h after finishing a competitive 1,000 mile race within less than nine days. Permeabilized fibers (0.81-1.28 mg ± 0.12 SD wet weight per assay) were prepared from needle biopsies and immediately studied by high-resolution respirometry [2] using 12 chambers in parallel (Oroboros Oxygraph-2k). Compared to human skeletal muscle fibers, the canine samples were more delicate to handle, highly sticky and appeared to be fragile, disintegrating to various degrees during substrate-uncoupler-inhibitor titration (SUIT) protocols in mt-respiration medium MiR06Cr. Two substrate-uncoupler-inhibitor titration protocols were applied (Fig. 1). SUIT1 emphasized substrate control with fatty acid oxidation (FAO) versus carbohydrate oxidation capacity, whereas the focus of SUIT2 was on coupling control with CI-linked substrates. Both protocols were designed to provide a common reference state of CI&II-linked ET capacity, in comparison to separate Complex I- and Complex II-linked substrate states (CI versus CII). | ||
CI&II-linked ET capacity was 262±41 pmol∙s<sup>-1</sup>∙mg<sup>-1</sup> Ww independent of the presence or absence of 0.2 mM octanoyl carnitine (FAO). This is the highest value so far reported for mammalian skeletal muscle. Top human endurance athletes have a CI&II-linked ET capacity approaching 200 pmol∙<sup>-1</sup>1∙mg<sup>-1</sup> Ww [3], compared to 153±19 pmol∙s<sup>-1</sup>∙mg<sup>-1</sup> Ww in competitive racing horses [4]. | |||
|mipnetlab=AT Innsbruck Oroboros, SE Stockholm Boushel RC, US OK Stillwater Davis MS, CA Vancouver Boushel RC, US CO Fort Collins Miller BF, US CO Fort Collins Hamilton K | |||
|mipnetlab=AT Innsbruck | |||
}} | }} | ||
== Affiliation == | == Affiliation == | ||
1- | 1-Oroboros Instruments, Innsbruck, Austria; 2-The Swedish School Sports Health Sc, Lindigovagen, Sweden; 3-College Health Human Sc, Colorado State Univ., Fort Collins, CO, US; 4-Land O’Lakes Purina Feed, St Louis, MO, US, 5Comparative Exercise Physiol Lab, Center Veterinary Health Sc, Oklahoma State Univ, Stillwater, OK, US; 5-D Swarovski Research Lab, Dep Visceral Transplant Thoracic Surgery, Medical Univ Innsbruck, Austria. – [email protected] | ||
== Figures == | == Figures == | ||
[[Image: | [[Image:MiPschool2015Greenville Laner Figure.jpg|left|500px]] Figure 1. Coupling/substrate control diagrams. Coupling states: LEAK, L; OXPHOS, P; ET-pathway or Electron transfer-pathway state, E. Substrate states differ in '''SUIT1''' and '''SUIT2'''. Octanoylcarnitine, Oct 0.2 mM; malate, M 0.5 mM (OctM: FAO); pyruvate; P 5 mM; glutamate, G 10 mM (PGM: CI); succinate, S 10 mM (CI&II); rotenone, Rot 0.5 µM (CII); residual oxygen consumption, ROX with malonate, 5 mM, and antimycin A, 2.5 µM. | ||
'' | |||
| Line 38: | Line 25: | ||
== References and Acknowledgement == | |||
Supported by K-Regio project MitoFit. | |||
#Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E (2014) Cytochrome c flux control factor as a quality criterion in respiratory OXPHOS analysis in canine permeabilized fibers. Mitochondr Physiol Network 19.13:63-4. | |||
#Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58. | |||
#Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. | |||
#Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D (2012) Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7:e34890. | |||
{{Labeling | |||
|area=Respiration, Instruments;methods, Comparative MiP;environmental MiP, Exercise physiology;nutrition;life style | |||
|organism=Dog | |||
|tissues=Skeletal muscle | |||
|preparations=Permeabilized tissue | |||
|topics=Cyt c | |||
|couplingstates=OXPHOS, ET | |||
|pathways=F, N, S, NS | |||
|instruments=Oxygraph-2k, O2k-Protocol | |||
|event=Oral | |||
}} | |||
Latest revision as of 18:36, 10 January 2022
| Comparative mitochondrial physiology: OXPHOS and ET capacity in permeabilized fibers of canine superathletes. |
Link:
Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E (2015)
Event: MiPschool Greenville 2015
Comparative mitochondrial physiology strongly relies on quantitative data sets for comparison of OXPHOS capacities and respiratory control patterns between species and tissues. Combination and interpretation of a wide variety of studies requires standardization of respiratory protocols, implementation of quality control criteria, and consistency of normalization. Previously, we described a reference method for the application of a cytochrome c threshold as exclusion criterion in mitochondrial OXPHOS analyses [1]. Alaskan sled dogs (N=6) were studied 72 to 120 h after finishing a competitive 1,000 mile race within less than nine days. Permeabilized fibers (0.81-1.28 mg ± 0.12 SD wet weight per assay) were prepared from needle biopsies and immediately studied by high-resolution respirometry [2] using 12 chambers in parallel (Oroboros Oxygraph-2k). Compared to human skeletal muscle fibers, the canine samples were more delicate to handle, highly sticky and appeared to be fragile, disintegrating to various degrees during substrate-uncoupler-inhibitor titration (SUIT) protocols in mt-respiration medium MiR06Cr. Two substrate-uncoupler-inhibitor titration protocols were applied (Fig. 1). SUIT1 emphasized substrate control with fatty acid oxidation (FAO) versus carbohydrate oxidation capacity, whereas the focus of SUIT2 was on coupling control with CI-linked substrates. Both protocols were designed to provide a common reference state of CI&II-linked ET capacity, in comparison to separate Complex I- and Complex II-linked substrate states (CI versus CII).
CI&II-linked ET capacity was 262±41 pmol∙s-1∙mg-1 Ww independent of the presence or absence of 0.2 mM octanoyl carnitine (FAO). This is the highest value so far reported for mammalian skeletal muscle. Top human endurance athletes have a CI&II-linked ET capacity approaching 200 pmol∙-11∙mg-1 Ww [3], compared to 153±19 pmol∙s-1∙mg-1 Ww in competitive racing horses [4].
• O2k-Network Lab: AT Innsbruck Oroboros, SE Stockholm Boushel RC, US OK Stillwater Davis MS, CA Vancouver Boushel RC, US CO Fort Collins Miller BF, US CO Fort Collins Hamilton K
Affiliation
1-Oroboros Instruments, Innsbruck, Austria; 2-The Swedish School Sports Health Sc, Lindigovagen, Sweden; 3-College Health Human Sc, Colorado State Univ., Fort Collins, CO, US; 4-Land O’Lakes Purina Feed, St Louis, MO, US, 5Comparative Exercise Physiol Lab, Center Veterinary Health Sc, Oklahoma State Univ, Stillwater, OK, US; 5-D Swarovski Research Lab, Dep Visceral Transplant Thoracic Surgery, Medical Univ Innsbruck, Austria. – [email protected]
Figures
Figure 1. Coupling/substrate control diagrams. Coupling states: LEAK, L; OXPHOS, P; ET-pathway or Electron transfer-pathway state, E. Substrate states differ in SUIT1 and SUIT2. Octanoylcarnitine, Oct 0.2 mM; malate, M 0.5 mM (OctM: FAO); pyruvate; P 5 mM; glutamate, G 10 mM (PGM: CI); succinate, S 10 mM (CI&II); rotenone, Rot 0.5 µM (CII); residual oxygen consumption, ROX with malonate, 5 mM, and antimycin A, 2.5 µM.
References and Acknowledgement
Supported by K-Regio project MitoFit.
- Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E (2014) Cytochrome c flux control factor as a quality criterion in respiratory OXPHOS analysis in canine permeabilized fibers. Mitochondr Physiol Network 19.13:63-4.
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58.
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45.
- Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D (2012) Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7:e34890.
Labels: MiParea: Respiration, Instruments;methods, Comparative MiP;environmental MiP, Exercise physiology;nutrition;life style
Organism: Dog
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Regulation: Cyt c Coupling state: OXPHOS, ET Pathway: F, N, S, NS HRR: Oxygraph-2k, O2k-Protocol Event: Oral