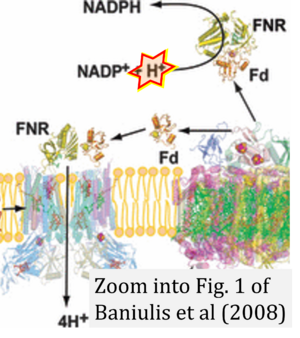
Baniulis 2008 Photochem Photobiol
| Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008) Structure-function of the cytochrome b6f complex. Photochem Photobiol 84:1349-58. https://doi.org/10.1111/j.1751-1097.2008.00444.x |
Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008) Photochem Photobiol
Abstract: The structure and function of the cytochrome b6 f complex is considered in the context of recent crystal structures of the complex as an eight subunit, 220 kDa symmetric dimeric complex obtained from the thermophilic cyanobacterium, Mastigocladus laminosus, and the green alga, Chlamydomonas reinhardtii. A major problem confronted in crystallization of the cyanobacterial complex, proteolysis of three of the subunits, is discussed along with initial efforts to identify the protease. The evolution of these cytochrome complexes is illustrated by conservation of the hydrophobic heme-binding transmembrane domain of the cyt b polypeptide between b6 f and bc1 complexes, and the rubredoxin-like membrane proximal domain of the Rieske [2Fe-2S] protein. Pathways of coupled electron and proton transfer are discussed in the framework of a modified Q cycle, in which the heme cn, not found in the bc1 complex, but electronically tightly coupled to the heme bn of the b6 f complex, is included. Crystal structures of the cyanobacterial complex with the quinone analogue inhibitors, NQNO or tridecyl-stigmatellin, show the latter to be ligands of heme cn, implicating heme cn as an n-side plastoquinone reductase. Existing questions include (a) the details of the shuttle of: (i) the [2Fe-2S] protein between the membrane-bound PQH2 electron/H+ donor and the cytochrome f acceptor to complete the p-side electron transfer circuit; (ii) PQ/PQH2 between n- and p-sides of the complex across the intermonomer quinone exchange cavity, through the narrow portal connecting the cavity with the p-side [2Fe-2S] niche; (b) the role of the n-side of the b6 f complex and heme cn in regulation of the relative rates of noncyclic and cyclic electron transfer. The likely presence of cyclic electron transport in the b6 f complex, and of heme cn in the firmicute bc complex suggests the concept that hemes bn-cn define a branch point in bc complexes that can support electron transport pathways that differ in detail from the Q cycle supported by the bc1 complex.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.