Cortez-Pinto 2009 J Hepatol
| Cortez-Pinto H, Machado MV (2009) Uncoupling proteins and non-alcoholic fatty liver disease. J Hepatol 50:857-60. https://doi.org/10.1016/j.jhep.2009.02.019 |
Cortez-Pinto H, Machado MV (2009) J Hepatol
Abstract: Non-alcoholic fatty liver disease (NAFLD) is a clinico-pathological syndrome characterized by lipid deposition in hepatocytes, in the absence of excessive alcohol consumption. It is now considered part of metabolic syndrome, with insulin resistance as a primary underlying derangement. It is widely accepted that a first hit leads to hepatic steatosis, and further hits to necro-inflammation and fibrosis (steatohepatitis), with oxidative stress, reactive oxygen species (ROS) and endoplasmatic reticulum stress playing a major role [1]. Mitochondrial dysfunction seems to be crucial in the pathogenesis of NAFLD, with less fatty acid oxidation favoring fat accumulation [2], and also being the major source of ROS contributing to necro-inflammation [3–5]. In fact, NAFLD has been considered a mitochondrial disease [6,7].
• Bioblast editor: Gnaiger E
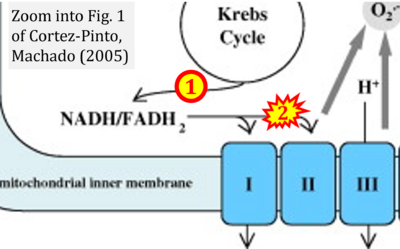
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase