Hanna 2023 Antioxid Redox Signal
| Hanna D, Kumar R, Banerjee R (2023) A metabolic paradigm for hydrogen sulfide signaling via electron transport chain plasticity. Antioxid Redox Signal 38:57-67. https://doi.org/10.1089/ars.2022.0067 |
Hanna D, Kumar R, Banerjee R (2023) Antioxid Redox Signal
Abstract: A burgeoning literature has attributed varied physiological effects to hydrogen sulfide (H2S), which is a product of eukaryotic sulfur amino acid metabolism. Protein persulfidation represents a major focus of studies elucidating the mechanism underlying H2S signaling. On the contrary, the capacity of H2S to induce reductive stress by targeting the electron transport chain (ETC) and signal by reprogramming redox metabolism has only recently begun to be elucidated. Recent Advances: In contrast to the nonspecific reaction of H2S with oxidized cysteines to form protein persulfides, its inhibition of complex IV represents a specific mechanism of action. Studies on the dual impact of H2S as an ETC substrate and an inhibitor have led to the exciting discovery of ETC plasticity and the use of fumarate as a terminal electron acceptor. H2S oxidation combined with complex IV targeting generates mitochondrial reductive stress, which is signaled through the metabolic network, leading to increased aerobic glycolysis, glutamine-dependent reductive carboxylation, and lipogenesis. Critical Issues: Insights into H2S-induced metabolic reprogramming are ushering in a paradigm shift for understanding the mechanism of its cellular action. It will be critical to reevaluate the physiological effects of H2S, for example, cytoprotection against ischemia-reperfusion injury, through the framework of metabolic reprogramming and ETC remodeling by H2S. Future Directions: The metabolic ramifications of H2S in other cellular compartments, for example, the endoplasmic reticulum and the nucleus, as well as the intersections between hypoxia and H2S signaling are important future directions that merit elucidation.
• Bioblast editor: Gnaiger E
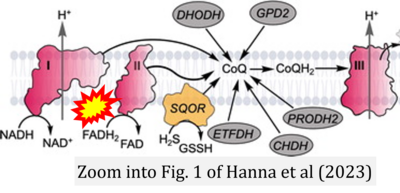
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase