Kataoka 2020 Microbiology Monographs
| Kataoka N, Matsutani M, Matsushita K (2020) Respiratory chain and energy metabolism of Corynebacterium glutamicum. In: Inui M, Toyoda K (eds) Corynebacterium glutamicum. Microbiology Monographs 23. Springer, Cham. https://doi.org/10.1007/978-3-030-39267-3_3 |
Kataoka N, Matsutani M, Matsushita K (2020) Microbiology Monographs
Abstract: The production of glutamic acid as well as amino acids of the aspartic acid family from carbohydrates is carried out industrially by a group of bacteria represented by Corynebacterium glutamicum. C. glutamicum is a Gram-positive facultative aerobe with a thick cell wall comprising, besides the peptidoglycan layer, a layer of mycolic acid and arabinogalactan. Industrially, glutamic acid production is induced by various stresses such as biotin limitation or the addition of surfactants or antibiotics, which may alter the cell membrane tension or opening of the mechano-sensitive channel as well as lead to the reduction of 2-oxoglutarate dehydrogenase activity that in turn affects the flow of the TCA cycle. Albeit the molecular events associated with these phenotypes are not fully understood, glutamate production in C. glutamicum is related to the cell surface structure (cell membrane) and the metabolic flux (especially through the TCA cycle) since these are deeply involved in cellular energetics. In the aerobic respiratory chain of C. glutamicum, several primary dehydrogenases, including type II NADH dehydrogenase, and malate:quinone oxidoreductase- and lactate dehydrogenase-dependent NADH reoxidizing systems, function donating electrons from each substrates to menaquinone, and the resulting menaquinol is oxidized by cytochrome bcc-aa 3 supercomplex and cytochrome bd oxidase. The bcc-aa 3 supercomplex and bd oxidase generate proton-motive force, H+/O ratio of 6 and 2, respectively. In this chapter, molecular characters and energetics of these respiratory components are summarized and also some metabolic engineering of C. glutamicum to produce valuable chemicals are described related to the respiratory chain.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
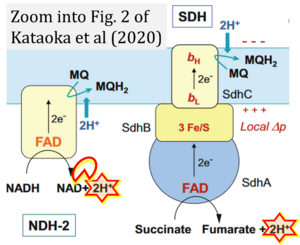
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.