Kim 2010 Korean Diabetes J
| Kim EH, Koh EH, Park JY, Lee KU (2010) Adenine nucleotide translocator as a regulator of mitochondrial function: implication in the pathogenesis of metabolic syndrome. Korean Diabetes J 34:146-53. https://doi.org/10.4093/kdj.2010.34.3.146 |
Kim EH, Koh EH, Park JY, Lee KU (2010) Korean Diabetes J
Abstract: Mitochondria play key roles in energy production and intracellular reactive oxygen species (ROS) generation. Lines of evidence have shown that mitochondrial dysfunction contributes to the development of metabolic syndrome. The causes of mitochondrial dysfunction are complex, but overnutrition and sedentary living are among the best known causes of mitochondrial dysfunction. ATP synthesized in the mitochondria is exchanged for cytosolic ADP by adenine nucleotide translocator (ANT) to provide a continuous supply of ADP to mitochondria. We recently found that ANT function is essential for peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1alpha)'s action on endothelial cells. PGC-1alpha is a transcriptional coactivator of nuclear receptors, playing an important role in fatty acid oxidation and mitochondrial biogenesis. Recent studies have shown that PGC-1alpha decreases intracellular ROS generation by increasing the expression of antioxidant genes. In our study, PGC-1alpha reduced cell apoptosis and ROS generation in endothelial cells by increasing ATP/ADP translocase activity of ANT and ANT1 expression. Here we review the role of ANT in maintaining proper mitochondrial function, and possible role of ANT dysfunction in the pathogenesis of metabolic syndrome.
• Bioblast editor: Gnaiger E
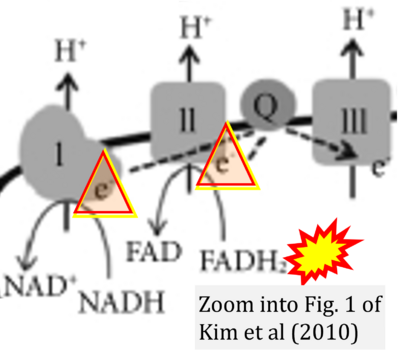
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase