Mathur 2017 Front Cell Neurosci
| Mathur D, Riffo-Campos AL, Castillo J, Haines JD, Vidaurre OG, Zhang F, Coret-Ferrer F, Casaccia P, Casanova B, Lopez-Rodas G (2017) Bioenergetic failure in rat oligodendrocyte progenitor cells treated with cerebrospinal fluid derived from multiple sclerosis poatients. Front Cell Neurosci 11:209. https://doi.org/10.3389/fncel.2017.00209 |
Mathur D, Riffo-Campos AL, Castillo J, Haines JD, Vidaurre OG, Zhang Fan, Coret-Ferrer F, Casaccia P, Casanova B, Lopez-Rodas G (2017) Front Cell Neurosci
Abstract: In relapsing-remitting multiple sclerosis (RRMS) subtype, the patient's brain itself is capable of repairing the damage, remyelinating the axon and recovering the neurological function. Cerebrospinal fluid (CSF) is in close proximity with brain parenchyma and contains a host of proteins and other molecules, which influence the cellular physiology, that may balance damage and repair of neurons and glial cells. The purpose of this study was to determine the pathophysiological mechanisms underpinning myelin repair in distinct clinical forms of MS and neuromyelitis optica (NMO) patients by studying the effect of diseased CSF on glucose metabolism and ATP synthesis. A cellular model with primary cultures of oligodendrocyte progenitor cells (OPCs) from rat cerebrum was employed, and cells were treated with CSF from distinct clinical forms of MS, NMO patients and neurological controls. Prior to comprehending mechanisms underlying myelin repair, we determine the best stably expressed reference genes in our experimental condition to accurately normalize our target mRNA transcripts. The GeNorm and NormFinder algorithms showed that mitochondrial ribosomal protein (Mrpl19), hypoxanthine guanine phosphoribosyl transferase (Hprt), microglobulin β2 (B2m), and transferrin receptor (Tfrc) were identified as the best reference genes in OPCs treated with MS subjects and were used for normalizing gene transcripts. The main findings on microarray gene expression profiling analysis on CSF treated OPCs cells revealed a disturbed carbohydrate metabolism and ATP synthesis in MS and NMO derived CSF treated OPCs. In addition, using STRING program, we investigate whether gene-gene interaction affected the whole network in our experimental conditions. Our findings revealed downregulated expression of genes involved in carbohydrate metabolism, and that glucose metabolism impairment and reduced ATP availability for cellular damage repair clearly differentiate more benign forms from the most aggressive forms and worst prognosis in MS patients.
• Bioblast editor: Gnaiger E
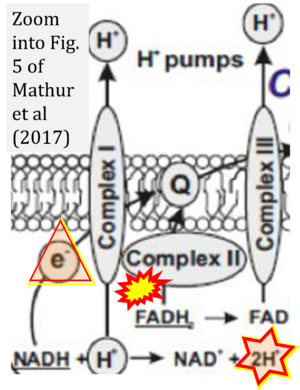
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 5 of Mathur et al (2017): H+ is consumed in the redox chemical (scalar) reaction of H+-linked electron transfer catalyzed by CI, NADH + H+ → NAD+ + 2H+. This same H+ is shown to be transported in the vectorial H+ translocation from the matrix side across the mtIM (H+neg → H+pos). The scalar and vectorial transformations must be distinguished. When the path is shown of the single electron (instead of 2{H++e-}) from NADH to ubiquinone, then it is particularly confusing when the 2H+ in the H+-linked electron transfer are indicated as remaining in the matrix.