Schoepf 2016 FEBS J
| Schöpf B, Schäfer G, Weber A, Talasz H, Eder IE, Klocker H, Gnaiger E (2016) Oxidative phosphorylation and mitochondrial function differ between human prostate tissue and cultured cells. https://doi.org/10.1111/febs.13733 |
» FEBS J 283:2181-96. PMID: 27060259 Open Access »![]()
Schoepf B, Schaefer G, Weber A, Talasz H, Eder IE, Klocker H, Gnaiger Erich (2016) FEBS J
Abstract: Altered mitochondrial metabolism plays a pivotal role in the development and progression of various diseases, including cancer. Cell lines are frequently used as models to study mitochondrial (dys)function but little is known about their mitochondrial respiration and metabolic properties in comparison to the primary tissue of origin. We have developed a method for assessment of oxidative phosphorylation in prostate tissue samples of only 2 mg wet weight using high-resolution respirometry. Reliable protocols were established to investigate the respiratory activity of different segments of the mitochondrial electron transfer-pathway in mechanically permeabilized tissue biopsies. Additionally, the widely used immortalized prostate epithelial and fibroblast cell lines RWPE1 and NAF, representing the major cell types in prostate tissue, were analyzed and compared to the tissue of origin. Our results show that mechanical treatment without chemical permeabilization agents or sample processing constitutes a reliable preparation method for OXPHOS analysis in small amounts of prostatic tissue typically obtained by prostate biopsy. The cell lines represented the bioenergetic properties of fresh tissue to a limited extent only. Particularly, tissue showed a higher oxidative capacity with succinate and glutamate, whereas pyruvate was a substrate supporting significantly higher respiratory activities in cell lines. Several fold higher zinc levels measured in tissue compared to cells confirmed the role of aconitase for prostate specific metabolism in agreement with observed respiratory properties. In conclusion, combining the flexibility of cell culture models and tissue samples for respirometric analysis are powerful tools for investigation of mitochondrial function and tissue specific metabolism.
• Keywords: Prostate metabolism, Mitochondria, Oxidative phosphorylation, electron transfer-pathway, High-resolution respirometry, Substrate-uncoupler-inhibitor-titration, RWPE1 fibroblasts, NAF fibroblasts
• Bioblast editor: Gnaiger E
• O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Oroboros
O2k-brief
SUIT protocols
Cited by
- 16 articles in PubMed (2022-06-12) https://pubmed.ncbi.nlm.nih.gov/27060259/
Labels: MiParea: Respiration, Comparative MiP;environmental MiP, mt-Medicine
Pathology: Cancer
Organism: Human Tissue;cell: Endothelial;epithelial;mesothelial cell, Genital, Other cell lines, Fibroblast Preparation: Permeabilized cells, Permeabilized tissue Enzyme: TCA cycle and matrix dehydrogenases
Coupling state: LEAK, OXPHOS, ET Pathway: F, N, S, NS, Other combinations, ROX HRR: Oxygraph-2k
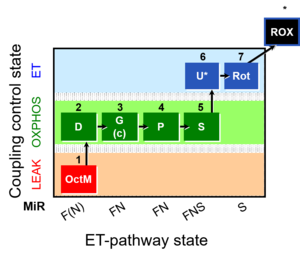
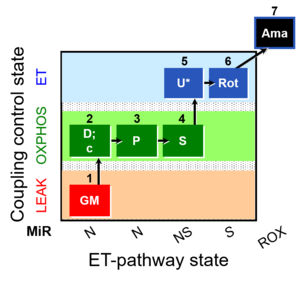
MitoFitPublication, 1OctM;2D;3G;4P;5S;6U;7Rot-, SUIT-015, 1GM;2D;2c;3P;4S;5U;6Rot-, SUIT-014, SUIT-015 O2 pti D043, O2k-brief