Vayalil 2019 Oncol Lett
| Vayalil PK (2019) Mitochondrial oncobioenergetics of prostate tumorigenesis. Oncol Lett 18:4367-76. doi: 10.3892/ol.2019.10785 |
Vayalil PK (2019) Oncol Lett
Abstract: Mitochondria are emerging as key players in the tumorigenic process of cells by maintaining the biosynthetic and energetic capabilities of cancer cells. It is now evident that mitochondria are involved in the malignant transformation, cell proliferation, aggression and metastatic behavior of prostate cancer (PC). Recent comprehensive analysis of the mitochondrial oncobioenergetic (MOB) profile of PC cells using microplate-based high resolution respirometry has clearly demonstrated that characteristic MOB alterations occur at different stages of PC development. Additionally, studies have reported that modification of the MOB profile significantly inhibits the growth of malignant cells. This observation suggests that dynamic alterations in the MOB function are a critical component in the development of malignant disease of the prostate. Therefore, quantification of MOB function may be a good marker for the prediction of tumor stage. Future studies may develop novel approaches to measure oncobioenergetics of tumors with minimal invasive procedures effectively in men to determine the general prostate health and tumor staging, and to predict whether a tumor will proceed to malignancy in patients. Additionally, since PC is a slow growing tumor, modulating the MOB profile at specific stages of tumor development may be a novel approach to treat or prevent PC.
• Bioblast editor: Gnaiger E
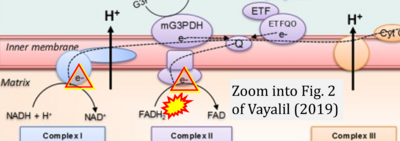
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase