Yang 2021 Springer
| Yang Y, Wu Y, Sun XD, Zhang Y (2021) Reactive oxygen species, glucose metabolism, and lipid metabolism. In: Huang C, Zhang Y (eds) Oxidative stress. Springer, Singapore. https://doi.org/10.1007/978-981-16-0522-2_9 |
Yang Y, Wu Y, Sun XD, Zhang Y (2021) Springer
Abstract: Reactive oxygen species (ROS) are free radicals produced by the reduction of molecular oxygen. The mitochondrial respiratory chain complex is regarded as a major ROS producer, which is composed of four protein complexes, the complex I, complex II, complex III, and complex IV. In recent years, a large amount of evidence has demonstrated that ROS plays a pivotal role in regulating cell glucose and lipid metabolism and participates in the occurrence and development of disorders related to glucose and lipid metabolism. Generally, glucose metabolism increases the production of ROS and enhances oxidative stress by glucose auto-oxidation and glycosylation of proteins and activated the polyalcohol pathway, etc. This review aims to clarify the role of ROS in regulating glucose metabolism (glucose uptake, glycolysis, gluconeogenesis, glycogen synthesis, glycogenolysis, pentose phosphate pathway).
As we all know, ROS plays a very important role in the pathogenesis of metabolic diseases induced by dysregulation of lipid metabolism, such as obesity, nonalcoholic fatty liver disease, and atherosclerosis. In this chapter, we focus on the interaction between ROS and lipid metabolism, especially the effects of ROS on the synthesis and oxidation of fatty acids, as well as phospholipids, cholesterol, and browning of beige fat.
• Bioblast editor: Gnaiger E
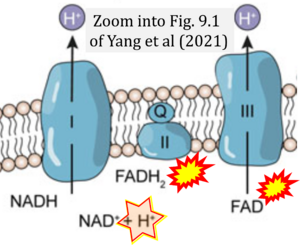
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 9.1 Arrows are missing from substrates to products, which are required to make this graph meaningful.