Lancaster 2001 FEBS Lett
| Lancaster CR (2001) Succinate:quinone oxidoreductases--what can we learn from Wolinella succinogenes quinol:fumarate reductase?. FEBS Lett 504:133-41. https://doi.org/10.1016/s0014-5793(01)02706-5 |
Lancaster CR (2001) FEBS Lett
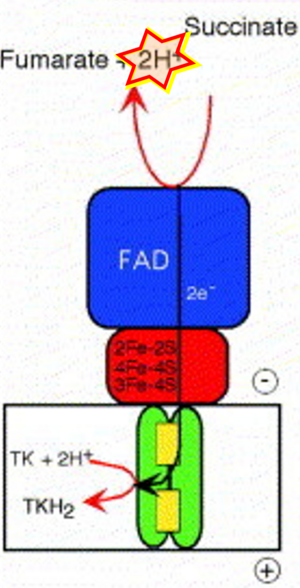
Abstract: The structure of Wolinella succinogenes quinol:fumarate reductase by X-ray crystallography has been determined at 2.2-A resolution [Lancaster et al. (1999), Nature 402, 377-385]. Based on the structure of the three protein subunits A, B, and C and the arrangement of the six prosthetic groups (a covalently bound FAD, three iron-sulphur clusters, and two haem b groups) a pathway of electron transfer from the quinol-oxidising dihaem cytochrome b in the membrane to the site of fumarate reduction in the hydrophilic subunit A has been proposed. By combining the results from site-directed mutagenesis, functional and electrochemical characterisation, and X-ray crystallography, a residue was identified which is essential for menaquinol oxidation. [Lancaster et al. (2000), Proc. Natl. Acad. Sci. USA 97, 13051-13056]. The location of this residue in the structure suggests that the coupling of the oxidation of menaquinol to the reduction of fumarate in dihaem-containing succinate:quinone oxidoreductases could be associated with the generation of a transmembrane electrochemical potential. Based on crystallographic analysis of three different crystal forms of the enzyme and the results from site-directed mutagenesis, we have derived a mechanism of fumarate reduction and succinate oxidation [Lancaster et al. (2001) Eur. J. Biochem. 268, 1820-1827], which should be generally relevant throughout the superfamily of succinate:quinone oxidoreductases.
• Bioblast editor: Gnaiger E
fumarate + 2H+ is ambiguous [1].
- 1. Gnaiger E (2023) Complex II ambiguities ― FADH2 in the electron transfer system. MitoFit Preprints 2023.3. https://doi.org/10.26124/mitofit:2023-0003.v4
Labels:
Enzyme: Complex II;succinate dehydrogenase