Difference between revisions of "Succinate"
| Line 2: | Line 2: | ||
|abbr=S | |abbr=S | ||
|description=[[File:Succinic_acid.jpg|left|100px|Succinic acid]] | |description=[[File:Succinic_acid.jpg|left|100px|Succinic acid]] | ||
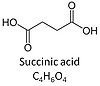
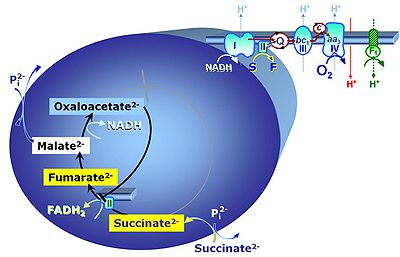
'''Succinic acid''', C<sub>4</sub>H<sub>6</sub>O<sub>4</sub>, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion '''succinate<sup>2-</sup>, S''', with ''p''K<sub>a1</sub> = 4.2 and ''p''K<sub>a2</sub> = 5.6. Succinate is formed in the [[TCA cycle]], and is a substrate of [[Complex II |CII]], reacting to [[fumarate]] and feeding electrons into the [[Q-junction]]. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the [[dicarboxylate carrier]]. The plasma membrane of many cell types is impermeable for succinate (but see [[Zhunussova 2015 Am J Cancer Res]] for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of [[oxaloacetate]], which is a potent inhibitor of Complex II (compare [[Succinate and rotenone]]). High activities of mt[[Malic enzyme]] (mtME) prevent accumulation of oxaloacetate. | '''Succinic acid''', C<sub>4</sub>H<sub>6</sub>O<sub>4</sub>, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion '''succinate<sup>2-</sup>, S''', with ''p''K<sub>a1</sub> = 4.2 and ''p''K<sub>a2</sub> = 5.6. Succinate is formed in the [[TCA cycle]], and is a substrate of [[Complex II |CII]], reacting to [[fumarate]] and feeding electrons into the [[Q-junction]]. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the [[dicarboxylate carrier]]. The plasma membrane of many cell types is impermeable for succinate (but see [[Zhunussova 2015 Am J Cancer Res]] for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of [[oxaloacetate]], which is a potent inhibitor of Complex II (compare [[Succinate and rotenone]]). High activities of mt-[[Malic enzyme]] (mtME) prevent accumulation of oxaloacetate. | ||
|info=[[Gnaiger | |info=[[Gnaiger 2019 MitoPathways]], [[Tretter 2016 Biochim Biophys Acta]] | ||
}} | }} | ||
{{MitoPedia topics | {{MitoPedia topics | ||
Revision as of 09:01, 12 August 2019
Description
Succinic acid, C4H6O4, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion succinate2-, S, with pKa1 = 4.2 and pKa2 = 5.6. Succinate is formed in the TCA cycle, and is a substrate of CII, reacting to fumarate and feeding electrons into the Q-junction. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the dicarboxylate carrier. The plasma membrane of many cell types is impermeable for succinate (but see Zhunussova 2015 Am J Cancer Res for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of oxaloacetate, which is a potent inhibitor of Complex II (compare Succinate and rotenone). High activities of mt-Malic enzyme (mtME) prevent accumulation of oxaloacetate.
Abbreviation: S
Reference: Gnaiger 2019 MitoPathways, Tretter 2016 Biochim Biophys Acta
MitoPedia topics:
Substrate and metabolite
Application in HRR
S: Succinate (Succinate disodium salt, hexahydrate, C4H404Na2 * (H2O)6)
- Sigma S 2378, 100 g, store at RT; FW = 270.1
- Preparation of 1 M stock solution:
- Weight 1.3505 g of succinate and dissolve in 3 mL H2O;
- Check pH and adjust to 7.0 if necessary with 1 M HCl (usually the pH is 7 without any adjustment);
- Transfer to 5 mL volumetric glass flask and adjust the final volume to 5 mL;
- Divide into 0.5 mL portions;
- Store frozen at -20 °C.
- O2k manual titrations MiPNet09.12 O2k-Titrations
- In the absence of CI-linked substrates, add the CI-inhibitor rotenone before addition of succinate, to avoid accumulation of oxaloacetate with subsequent inhibition of succinate dehydrogenase. See: Succinate and rotenone.
- When keeping the succinate stock solution on ice, check for complete solubilization of succinate and warm the stock solution in your hands if necessary.
- Titration volume: 20 µL using a 50 µL syringe (2 mL O2k-chamber).
- Final concentration: 10 mM.
SUITbrowser question: Succinate pathway
- The SUITbrowser can be used to find the best SUIT protocols to analyze the succinate pathway, among other research questions.