Talk:O2k-pH ISE-Module
Introduction and Scope
The O2k Core / O2k-MultiSensor extension of the OROBOROS Oxygraph-2k yields a record of a potentiometric (voltage) signal simultaneously to the oxygen signal in both chambers of the O2k. A new pH probe system is described here, consisting of separate reference and measuring electrodes.
Terms used:
pH/ISE: The potentiometric channels may be used with different kinds of pH / ISE (Ion Selective Electrode) systems: With the pH / reference electrode system described here, or with an ISCE – an ion selective combination electrode, combining reference and measuring electrode into one body (most pH electrodes) or with an ISE system for other ions (TPP+, Ca2+,...), see O2k-TPP+_and_Ca2+_ISE-Module. The O2k-Core and MultiSensor extensions for Oxygraph Series D (and higher) not only include the two potentiometric channels, but contain two a additional amperometric (current) channels for the measurement of NO (H2O2, etc). Such amperometric measurements are also possible with an O2k Series A to C by using one of the oxygen channels together with an OROBOROS NO-Preamplifier (no MultiSensor extension required). These amperometric O2k-MultiSensor applications are discussed separately in “O2k-Manual: Amperometric Electrodes” H MiPNet15.05 NO-Manual.
pX Potentiometric measurements result in a voltage signal which is typically a linear function of the logarithm of the activity (concentration) of the substance of interest (the analyte). A calibrated pH electrode displays the negative decadic logarithm of the H+ ion activity (potentia hydrogenii) and thus got its name “pH electrode”. By analogy, an ISE may be used to measure pTPP, pCa, etc., hence the general term “pX” is used to denote the signal from such an ISE.
The pH System
'
Unlike previous models the pH set can be stored as received before use. However, please check immediately after receiving the set for transport damage (broken glass parts) and whether the seal of the vial with the pH electrode is still intact.
However, please consider that all glass pH electrodes have a limited lifetime even when not used, see below.
The pH electrode: Shipping, Storage and Maintenance
The reference electrode is shipped in a dry state and has to be filled before use, see below. The pH electrode is shipped with the glass bulb immersed in a vial containing a wet sponge.The pH electrode can be stored in this condition. Carefully unwind the tape and remove the probe from the protective glass tube.
Conditioning: Optimum response time will be obtained after the probe has been exercised in two buffer solutions. Place a pH 4 buffer or equivalent in a beaker and a pH7 buffer or equivalent in a second beaker. Hold the pH electrode and reference electrode together and touch the pH4 buffer surface, allowing 15-20 seconds of equilibration. Rinse the two electrodes with distilled water and then touch the pH 7 buffer surface in the same manner. Do this several times.
Handling: Be careful not to apply pressure against, or to shock, the inner glass capillary tube!
Cleaning: When using the electrode in solutions containing protein, the electrode and reference electrode should be soaked in enzyme cleaning solution such as Terg-a-zyme, by Alconox Inc, or a chromic/sulfuric acid glass cleaning solution after each use for a few minutes to remove the protein from the glass and the reference junction. This will prolong the life of the electrode.
Storing: Always clean the electrode before storing.
Long-term (over 2 weeks): Return the electrode to its original container and prepare it in the same condition in which your received it. Usually this means moistening the sponge located in the bottom of the protective glass tube with distilled water.
Short-term: The electrode can be left in acidic pH buffer solution, e.g. pH4.
Troubleshooting: If possible try to locate the problem either at the measuring pH or at the reference electrode by switching electrodes. If you have only one reference electrode you can switch to a spare glass barrel for diagnostic purposes. The following text assumes that the problem was located on the reference electrode.
Little or no response: Inspect the electrode for visible cracks (usually occurring at the tip of the electrode). If any exists, the electrode is defective and must be replaced. The slightest crack in or around the tip of the electrode may cause the electrode to read about the same in all solutions.
Response pegs OFF Scale: 1.) Check the pX gain setting.
2.) Visually inspect the electrode for a broken bulb.
Sluggish Response: If the electrode becomes sluggish in responding to changes in pH, the response time can be improved using the following procedure:
First: Clean the electrode as described earlier.
Second: Soak the electrode in 0.1 N HCl for 5 minutes, followed by soaking in 0.1 N NaOH for 5 minutes. After repeating several times, rinse the electrode thoroughly with distilled water. The electrode can then be calibrated in the usual manner.
Reference Electrode: Assembly, Storage and Maintenance
The electrode is composed of an internal silver-silver chloride electrode with an internal filling solution of 3 M KCl saturated with AgCl. Before the electrode can be placed into operation, the glass reference barrel must be filled with the internal reference solution supplied.
Filling the reference barrel:
- Unscrewing the white plastic cap: Remove the upper part of the cap with the attached silver wire. Pull the glass out of the lower part of the cap.
- The internal reference solution is added to the glass tube using the provided electrolyte bottle and polyethylene tubing: Insert filling tube into nipple of electrolyte bottle. Push until tube locks into place. Insert tube into reference barrel and squeeze bottle. Fill reference barrel up to approximately 1.5 cm (approx. 0.5 inch) from top.
- After filling the glass barrel with the reference electrolyte, the silver wire is inserted into the glass tube and the electrode cap is re-assembled.
Cleaning the electrode: To wash the reference electrode between runs, rinsing is recommended in the sequence water, pure ethanol, and water. This procedure should be usually sufficient to prevent carry-over even of hydrophobic inhibitors, since the reference electrode is made of non-hydrophobic materials. Immersion into pure ethanol for longer periods of time should be avoided to prevent blocking of the ceramic diaphragm in an assembled electrode. When using the electrode in solutions containing higher concentrations of protein, the electrode should be soaked in a dedicated enzyme cleaning solution or a chromic/sulfuric acid glass cleaning solution after each use for 10-15 seconds to remove the protein from the glass and the reference junction. This will prolong the useful life of the electrode.
Storing the electrode: Always clean the electrode before storing. Protect reference electrodes from light during storage, e.g. by wrapping them in aluminum paper.
Short term: Place the tip of the electrode in a test tube or beaker containing reference electrolyte (3 M KCl). Falcon type 15 ml vials are well suited. If necessary refill electrolyte before use.
Long-term (>4 weeks): Remove the glass barrel containing the electrolyte and store the entire glass barrel in a closed test tube filled with the reference electrolyte. Rinse the silver wire and electrode cap to remove the salt solution and dry using an absorbent towel. Store in the accessory box or any closed container to keep dust off of the electrode and protect from light.
Troubleshooting: If possible try to locate the problem either at the measuring pH or at the reference electrode by switching electrodes. If you have only one reference electrode you can switch to a spare glass barrel for diagnostic purposes. The following text assumes that the problem was located on the reference electrode.
Little or no response: Inspect the electrode for visible cracks. If any exists, the glass barrel is defective and must be replaced with a spare. The slightest crack in or around the tip of the electrode may cause the electrode to read about the same in all solutions.
Response pegs OFF Scale: 1.) Check the pX gain setting.
2.) Visually inspect the electrode for broken or dissolving internal Ag-AgCl wire or for inadequate volume of reference electrolyte. Reference electrolyte level should be above the Ag-AgCl element.
3.) Blocked or clogged liquid junction – clean electrode tip first then soak the tip of the electrode in warm (not hot) distilled water for 5 to 10 minutes. If still clogged, then soak overnight in distilled water or replace reference barrel with spare barrel supplied.
O2k-MultiSensor System
Applications of the O2k with one or two potentiometric electrodes (ISE or pH) require an O2k-Core or an instrument with electronic MultiSensor upgrading. In addition to the two polarographic (amperometric) oxygen channels of the basic O2k, the O2k-Core and the MultiSensor system provide two electronic channels for potentiometric (voltage) measurements with ISE or pH (O2k Series B upwards). The Multisensor function of O2k Series D (and higher) is extended further with two additional amperometric channels (current measurement; for NO, etc.). Please see O2k-Main Unit#O2k-Series for how to determine the series of an oxygraph.
O2k-Core and O2k Series D (and higher)
Connect:In O2k Series D (and higher), pH and reference electrodes are directly connected to the plugs on the front side of the Oxygraph-2k. Insert the connector of the pH electrode into the BNC plug labelled “pX” and the connector of the reference electrode to the 2 mm pin plug labelled “Ref”, see MiPNet12.06.
Gain: The gain of the pX channel can be selected in the DatLab software in the Oxygraph-2k configuration window. For measurements with the OROBOROS pH system, a gain of 20 is suggested. Usually it will not be necessary to change the gain for pH work.
O2k Series B and C, pX Upgrade Installed Before 2011
Connect:To connect two electrodes (pH + reference electrode) to the MultiSensor BNC plug of the O2k (Series B and C), a MultiSensor Connector has to be installed. The MultiSensor Connector has a black cable with a male BNC plug and a 2 mm plug on its instrument side (facing the O2k), and a female BNC plug and a 2 mm plug on the opposite side for connecting the electrodes. Additionally, an Allen key and a cable with a 2 mm pin and a spade terminal are included in the MultiSensor Connector set.
First this additional cable should be attached to the Oxygraph-2k housing. Loosen one of the lower screws on the front side, bottom panel of the O2k (using the supplied Allen key), insert the connection of the thin black cable (spade terminal, red) and tighten the screw again. This additional cable provides a grounding connection to the O2k which improves signal stability. The cable can be left attached to the Oxygraph even when the MultiSensor Connector is not in use and not attached to the O2k.
For measurements connect the black cable of the MultiSensor Connector with the BNC plug to the multi-sensor BNC port of the Oxygraph, marked “pX”. Then plug the 2 mm pin of the cable attached to the Oxygraph housing into the 2 mm plug of the MultiSensor Connector that is situated on the same side as the black cable. To have the BNC cable and the 2 mm plug in correct positions versus the Oxygraph just turn the MultiSensor Connector over, if necessary. The reference electrode is then connected to the front side 2 mm plug and the measuring electrode (pH) to the front side BNC plug of the MultiSensor Connector.
Gain: The gain and offset of both potentiometric MultiSensor channels can be adjusted by turning the O2k housing on its side. At the bottom of the Oxygraph-2k there are 4 adjustable screws and a label indicating their functions.
Turning the screws clockwise increases; counter clockwise decreases gain and offset settings. For pH measurements between pH 4 and 10 it should not be necessary to change these settings. However, the settings might have been changed before for work with a different electrode. If you have set up your system and get a raw voltage beyond +9 or -9 volts, decrease the gain by one or two counter clockwise turns of the screw, repeat if necessary. Importantly, the raw voltage displayed in DatLab is already the amplified signal. It is usually not necessary to change the offset setting.
O2k Series B and C, pX Upgrade Installed After Jan 01, 2011
Connect:For O2k Series B to C with a pX upgrade installed after 2010, pH and reference electrodes are directly connected to the plugs on the front side of the Oxygraph-2k housing, as described above for O2k Series D (and higher). No special MultiSensor Connector is needed for such instruments. Insert the connector of the pH electrode into the BNC plug (nearest to the chamber) and the connector of the reference electrode to the 2 mm pin plug beside the BNC plug [[#manual MiPNet12.06]].
Gain: The gain of both potentiometric MultiSensor channels can be adjusted by turning the O2k housing on its side. At the bottom of the Oxygraph-2k there are 2 rotating switches and a label indicating their functions. The gain can be set to 10, 20, 40, or 80. The factory setting is a gain of 20. Usually a gain of 20 will also be very suitable for pH measurements. For some specific, extremely high-resolution pH measurements a higher gain might be advantageous to avoid limitation of resolution by digital noise. It is expected that for most users there never will arise the necessity to change the gain setting.
If you have set up your system and get a raw voltage beyond +9 or -9 volts, decrease the gain. Importantly, the raw voltage displayed in DatLab is already the amplified signal.
Operation Instructions
Insertion of Electrodes: Bubble-Free Filling of the Chambers
MultiSensor vs. Standard stoppers: The introduction of several (large) electrodes into the O2k-chamber through the stopper requires the use of a special “MultiSensor stopper”. The standard stopper has a concave shape on its end inserted into the chamber, with a single capillary (gas-escape/titration capillary) in the centre of the stopper (the highest point when inserted). The end of the pH-MultiSensor stopper is angular with one capillary and two electrode inlets. The gas-escape/titration capillary is at the side of the stopper at the highest point when inserted.
Preventing bubbles: When inserting the stopper into the O2k-chamber filled with aqueous medium, gas bubbles are guided into the gas-escape/titration capillary and pushed out of the chamber. This is more effective, however, with the standard stopper than the MultiSensor stopper. Therefore, great care should be taken to avoid the trapping of bubbles during initial insertion. The single most important point for prevention of bubble formation is to close the chamber only after full thermal equilibrium has been established. The best criterion for thermal equilibrium is a stable oxygen signal, with a slope near zero in the “open chamber” configuration used for oxygen sensor calibration, see MiPNet12.08.
- Fill the chamber with medium (2.6 ml for a 2 ml chamber) allowing for a well-defined air space when stirred (see [#section42 Section 4.2]).
- Place the stoppers on top of the chambers but do not yet close them. Activate stirring. A gas phase similar to the one for air calibration has to be visible. Using Graph layout “1. Calibration Gr3 Temp.”, wait until temperature, Peltier power, and oxygen concentration are stable and the slope of oxygen concentration is near zero (±1 pmol∙s1∙ml1).
- Calibrate the oxygen signal (air calibration), see MiPNet12.08.
- Stop the stirrers, and insert the stoppers completely into the chambers.
- Insert the reference electrode into slightly larger mm) inlet of the stopper (it won’t fit into the more narrow inlet, so it is a good idea always to start with the reference electrode to find to correct inlets). If a gas bubble remains in the chamber (but liquid is on top of the stopper) try to remove the gas bubble: inserting a short needle (if possible with a flat tip) without an attached syringe into the titration port usual removes any bubbles from the inlet, thereby allowing the big bubble to escape from the chamber. Smaller bubbles may be brought nearer to the gas-escape capillary by starting and stopping the stirrer several times. It may be necessary to lift the entire stopper (including pH electrode) to a position above the liquid phase and insert it again.
- Make sure that the smaller inlet for the reference electrode is totally filled with liquid – if necessary add more pre-warmed liquid (same composition as in the chamber) to the top of the stopper.
- Insert the reference electrode into the chamber. Move it up and down to get rid of any bubbles that might be trapped in its inlet. Switch on the stirrer and check for any bubbles. If there are bubbles, repeat the instructions described above.
- Connect the electrodes to their proper plugs, see above.
- Aspirate all excess liquid from the top of the stopper, making sure the top is dry and no liquid film connects the different inlets.
The uncorrected slope of the oxygen concentration should now be in the usual range for a closed chamber at atmospheric saturation (2 - 4 pmol∙s1∙ml1). Considerably different fluxes may indicate that there is a liquid “bridge” on top of the stopper connecting at least two different inlets, allowing the circulation of liquid between the chamber and the top of the stopper.
Volume Calibration for MultiSensor Stoppers
When using an MultiSensor stopper, the pH and reference electrodes must be in place when calibrating the O2k-chamber volume. This is similar to volume-calibration with standard stoppers, see MiPNet12.06.
- Add to the dry O2k-chamber containing the stirrer bar a water volume accounting for the final chamber volume (2 ml) plus the additional dead volume in the capillary and spaces between electrodes and inlets. For the OROBOROS pH Assembly (ion selective electrode + reference electrode), this additional volume is approximately 0.14 ml. Therefore, the necessary volume to calibrate a chamber volume of 2 ml with the OROBOROS pH system is 2.14 ml.
- Start stirring, cover the chamber with a Perspex cover or a loosely placed stopper, and wait for equilibration. To avoid creating bubbles during the calibration process it is very important to allow for full thermal equilibration of the liquid in the chamber. Continue with volume-calibration only after reaching the conditions for oxygen calibration at air saturation (stable temperature and Peltier power, near-zero uncorrected oxygen flux (±1 pmol∙s1∙ml1).
- Prepare the MultiSensor stopper as described for a standard stopper (loosening the calibration ring, drying the stopper), making sure that the three inlets are dry. Remove the pH and the references electrode from their respective storage solutions. Dry their shafts with a paper towel (do not use a paper towel directly on glas bulb of the pH electrode or the diaphragm of the reference electrode). Insert the electrodes into the MultiSensor stopper.
- Stop the stirrer. Place the stopper on top of the chamber with a loosened volume-calibration ring slid down to the chamber holder. Insert the MultiSensor stopper slowly into the unstirred chamber carefully observing first the diminishing gas phase in the chamber. Then focus on the top of the stopper. Stop the insertion as soon as the first drop of liquid appears on the top of the stopper. This may be visible first on top of the gas-ejection capillary comparable to the standard stoppers, but it may also occur at the edge of the reference electrode or the pH electrode.
- Fix the position of the volume calibration ring by tightening the screw as in the procedure with a standard stopper.
Handling Issues During an Experiment
Two problems have to be avoided while running an experiment with a MultiSensor stopper:
(a) Introduction of bubbles: After the chamber was filled as described, no gas bubbles should be either in the chamber or in the capillary.
(b) Circulation of liquid between the top of the stopper and the internal chamber needs to be prevented by aspirating any excess liquid form the top of the stopper. These conditions have to be maintained during the entire experiment, removing excess liquid from the stopper after any titration.
Injections: Before inserting a syringe needle into the stopper (manual or TIP syringe), make sure that the capillary is filled with liquid – if necessary, place a drop of liquid on top of the capillary - then remove any bubbles from the capillary by using a needle without an attached syringe. A gas-escape/titration capillary filled with liquid without any gas bubbles provides good visibility through the capillary to the light within the chamber. If you cannot see the light, the capillary is blocked by gas bubbles. These need to be removed. Similarly, when the stirrer is switched off, an internally trapped gas bubble might move into a position to block the light, which can be checked further by switching the stirrer on and off.
Insert the needle and perform the titration (manual or TIP2k). After removing the needle, remember to aspirate any excess liquid from the top of the stopper that has been ejected from the constant-volume chamber during titration. It is important to minimize the time span during which a liquid bridge exists between the different inlets through the stopper.
Instrumental Background Oxygen Flux
Instrumental oxygen background parameters are used to correct on-line biologic oxygen flux [[#protocol MiPNet14.06]]. Instrumental background tests have to be carried out with the MultiSensor stopper and all electrodes in place. Instrumental background parameters obtained with standard stoppers cannot be used to calculate biological oxygen consumptions obtained in a MultiSensor experiment.
4.4.1. Dithionite Background
Because of difficulties involved in opening and closing the O2k-chamber closed with a MultiSensor stopper and electrodes, it is strongly recommended to use the instrumental background procedure based on dithionite injections [[#protocol MiPNet14.06]]. This method avoids repeated opening and closing of the O2k-chamber. To run an instrumental oxygen background experiment, set up the chambers and electrodes as described above and then follow the procedure see MiPNet14.06.
4.4.2. Instrumental Background Parameters for Oxygen Flux
An O2k-chamber with a MultiSensor stopper has a higher oxygen back diffusion, a0 at zero oxygen concentration, as compared with a standard stopper. In a 2 ml chamber using the OROBOROS pH system in MiR06 at 37 °C, with an oxygen regime from air saturation to low oxygen, the backdiffusion parameter, a°, typically ranges from -4 to -8 pmol∙s1∙ml1. If more negative fluxes (< -10 pmol∙s1∙ml1) are detected in the background experiment, this is a strong indication that a liquid bridge exist on the top of the stopper. This problem can be solved by simply aspirating any excess liquid from the top of the stopper.
pH Calibration
For how to calibrate the pH electrode using DatLab, see Manual “Oxygen and pX Calibration DatLab 4.x”, MiPNet 12.08. For more information about the calibration process see the protocol “pH Calibration” (“Measurement and Temperature Dependence of pH”, see MiPNet08.16 pH-Calibration.
Measuring Proton Production
From the Change of the pH Signal
To calculate the actual proton flow in the system from the observed change in the pH signal the buffering capacity of the medium has to be determination of before the introduction of sample. This is best achieved by setting up the TIP with HCl in the syringes and simulate the expected proton production of the sample by setting an appropriate flow rate for the TIP. In the simplest implementation just one flow rate is used for about 5 minutes. From the first time derivative of the pH signal the buffering capacity can be calculated. Afterwards the TIP has to be re-fitted with syringes containing KOH (NaOH) for using the pH-Stat during the actual biological experiment. The observed rates of pH changes during the biological experiment (pX slope/ first time derivative of pH signal) can then be directly converted to Proton flow values using the buffering capacity determined before. This is shown in the spreadsheet file "pH Stat Template Buffer Capacity_one_point" In a more sophisticated approach a multiple point calibration is done: Several proton flows are simulated before the experiment, a linear regression between set proton flow and observed pH slope is done and the regression parameters are used to calculate proton flows from the pX slopes observed during the biological experiment.
These approaches assume a linear relationship between pH change and introduced protons. This is an approximation that is only valid for very small pH changes. In other words the buffering capacity has to be constant during the entire experiment.
Using DatLab 5
Proton production rates can be calculated on line. In the menu select [Plots]/[Proton Flux].
- Determining the buffering capacity of the medium:
- calibrate the pH electrode, observe the calibrted pH signal
- fill TIP syringes with diluted acid or base
- start a slow injection of acid or base into the media (no sample present)
- place a mark on a stable region of the slope plot of the calibrated pH signal 'pX slope"
- go to [Plots]/[Proton Flux]
- The buffering capacity is calculated by DatLab and can be used for calculations of biological proton flow in the same file or noted down and used in subsequent experiments.
- Biological proton flux
- calibrate the pH electrodes
- observe the pH calibrated signal
- place marks on regions of interest on the pX slope plot. If you use the "pH" stat" se above to keep the pH value in desired limit make sure that you exclude times during which base was injected
- select [Plots]/[Proton Flux]
- enter the buffering capacity in the appropriate field or use the feature in the upper part of the window to calculate the buffering capacity from a calibration experiment in the same file
- press [ok]
- anew plot "Proton Flux" is now available in [Graph]/[Select Plots] (right at the end of the list. You can now chose to display this plot e.g. instead of the pX slope plot by selecting its check box and de-selecting the check box of the "pX slope" plot.
Known issues: DatLab always calculates a new buffering capacity from the input in the upper part of the window and does not remember the value from previous files. Therefore, if the determination of buffering capacity was done in a different Datlab file the value has to manually entered.
From the Amount of Injected Base
The limitations mentioned above can be overcome by using the amount of base necessary in the pH-Stat approach to hold the pH value constant. If the pH values at the beginning and at the end of a time interval are identical then the proton floe during this time interval can be directly calculated from the amount of base injected to keep the pH value constant. While this method does not assume a constant buffering capacity during the entire experiment there are some drawbacks:
- Usually only the "pH-Stat-strict" approach will assure the identical pH values at the beginning and the end of a time period necessary for this approach (This could possibly be overcome by more advanced data analysis).
- Only one value for proton flow for each period between injections is calculated in contrast to the continuous recording facilitated by the first mentioned method.
- The injected volumes have to be read out form TIP events. This can be partially automated in a spreadsheet template (pH Stat Template Injected Volume) but is more tedious than the method using the buffering capacity
- Initial trials with simulated proton flows indicted this method to be slightly less precise than the method using the buffering capacity.
Conclusion and Templates
A potential compromise would be to use the method based on buffering capacity for routine calculations but check selected (late) phases of the experiment with the method based on added base volume. Thereby, significant changes in buffering capacity during the experiment should be detected. Spreadsheet (Excel) templates for both methods are available for download here.
Performance and Trouble Shooting
Performance criteria can be assessed by two tests:
1.Calibrations must be reproducible: After performing a two point calibration, reinserting the electrodes in the first used calibration buffer should give the correct calibrated pH value. The slope and intercept of the calibration can be copied to a spreadsheet file (sheet “pH calibration” in ”O2k-Calibration-List”) to obtain a track record..
2.Drift: The drift in the medium without biological sample hast to be small as compared to pH changes expected from the sample. The drift will be different in different media (buffering capacity), so for each used system the necessary experience has to be gained. The drift is the first time derivative of the pH signal, so in DatLab it will be shown as “pX Slope”. After proper calibration the unit of this plot will be mpH/s.
Trouble shooting: If the required performance criteria are not reached, the following steps should be tested:
- Set the polarisation voltage of the O2 sensor to 0 in the O2k Control window. Observe any effects on the pX raw signal. A tiny potential jump is acceptable. If a drift in the pX signal is either increased or reduced by this test or an extreme jump in the signal observed, the membrane of the polarographic oxygen sensor (POS) should be replaced. Reset the polarisation voltage to 800 mV after the test.
- If you have two pH electrodes: Locate the problem to either the reference electrode or the pH electrode by switching either only the pH or only the reference electrode between chambers.
- Follow the specific trouble shooting procedures for the reference electrode or the pH electrode described above in and .
pH electrode lifetime: All glass pH electrodes do have a limited lifetime due to aging of the glass membrane. After 1.5 to 2 years a loss of performance will occur even without use.
MultiSensor Features of DatLab 4
Observing the pX Signal
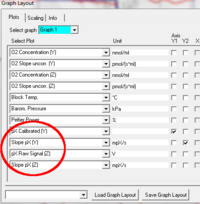
Graph layout: Three plots are available in DatLab based on the recorded pX signal: pX Raw Signal, pX Calibrated, and Slope pX. These plots can be selected from the drop-down lines and displayed with their check boxes either on the Y1 or Y2 [Graph layout / Select Plots].
pX Raw Signal displays the voltage between pH and reference electrode as recorded by the Oxygraph at a given gain setting.
pX Calibrated is the signal after calibration with the parameters set in the MultiSensor Calibration window.
Slope pX is the negative time derivative of the calibrated pX signal, multiplied by 1000, in units [mpX/s].
Graphs can be constructed to include both recorded oxygen and pX, or several graphs can be added to display oxygen and pX data separately. Some layout templates are provided, which can be modified and saved as appropriate. All graph settings can be saved as user-defined layouts, see [C MiPNet12.07 DatLab4-Guide.
The Oxygraph Window
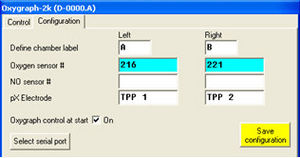
In the Configuration Table of the Oxygraph Control window, the used pX electrode can be entered for documentation purposes.
In the Control Table (O2k Series D and upwards) of the Oxygraph Control window, the gain for the pX channel can be set in the section “pX” to 10, 20, 40, or 80. The gain influences the “pX Raw Signal” recorded in DatLab. Therefore, a gain of 1 will give the same voltage [V] as would be measured with any multimeter between reference and measuring electrode. See MiPNet12.06, for a full screenshot of the Control table.
The MultiSensor Menu
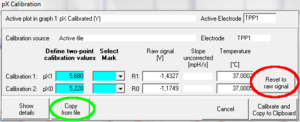
From the MultiSensor menu the MultiSensor Calibration window [Ctrl+F5] is opened This feature allows for a simple two-point linear calibration of pX (or –pX) as a function of recorded voltage, using known pX values, see “Oxygen and pX Calibration DatLab 4.x”, D MiPNet12.08 O2k-Calibration
Calibrations for different signal types: There is only one set of calibration values for each pX channel, irrespective of the connected electrode. If a pX channel was calibrated for a pH electrode, these values will initially also be used to calculate the calibrated signal when the pH electrode is exchanged for a TPP+ electrode. Even when observing only the raw (not the calibrated) signal, the time derivative (Slope pX) will be calculated from the calibrated signal, which might lead to confusion when the time derivative is used to access stability or signal drift. It is, therefore, suggested to set the calibrated signal to the raw signal whenever the raw signal is to be used as the primary data source.
When previous calibration settings are needed later (e.g. the channel is now again used with a pH electrode), the old calibration values can simply be restored by using the Copy from file button in the calibration window and select the file in which the original calibration (e.g. pH) was performed initially or a file in which these values were applied. Then calibrate with Calibrate and Copy to Clipboard.
Reset To Raw Signal: It is often desirable to set the calibrated signal equal to the raw signal. This can be done any time by pressing the Reset to raw signal button in the MultiSensor Calibration Window and calibrating with Calibrate and Copy to Clipboard.
References
O2k-Manual
Protocols