Difference between revisions of "Kabir 2014 Abstract MiP2014"
| Line 1: | Line 1: | ||
{{Abstract | {{Abstract | ||
|title=Declining follicular reserve triggers mitochondrial pathway of granulosa cell apoptosis and accelerates the rate of follicular decay. | |title=Declining follicular reserve triggers mitochondrial pathway of granulosa cell apoptosis and accelerates the rate of follicular decay. | ||
|info=[[File: | |info=[[File:Kabir_SN.jpg|240px|right|Kabir SS]] [http://www.mitophysiology.org/index.php?mip2014 MiP2014], [[Laner 2014 Mitochondr Physiol Network MiP2014|Book of Abstracts Open Access]] | ||
|authors=Banerjee S, Saraswat G, Kabir SN | |authors=Banerjee S, Saraswat G, Kabir SN | ||
|year=2014 | |year=2014 | ||
Revision as of 16:18, 11 August 2014
| Declining follicular reserve triggers mitochondrial pathway of granulosa cell apoptosis and accelerates the rate of follicular decay. |
Link:
MiP2014, Book of Abstracts Open Access
Banerjee S, Saraswat G, Kabir SN (2014)
Event: MiP2014
The ovary receives a finite pool of follicles during fetal life. The follicular reserve declines at an exponential rate leading to an accelerated rate of decay during the years preceding menopause [1]. The present investigation examined if diminished follicle reserve, which characterizes ovarian aging, impacts the attrition process.
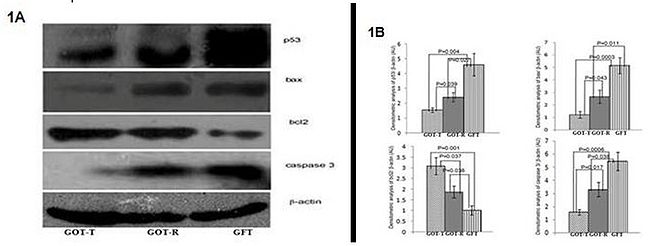
Premature ovarian aging was induced in rats by intra-embryonic injection (3 µl) of 100 µg of galactosyltransferase-antibody (GalTase-Ab) per embryo on D10 of pregnancy. The size of the follicle pool of post-natal D35 female rats was subsequently modulated by transplantation of either a wedge of fat (sham control) or an ovary from 25-day old control rats under the ovarian bursa. The ovaries were dissected out on post-natal D55. Follicular growth and atresia and ovarian microenvironment were evaluated by real-time RT-PCR analysis of growth differentiation factor-9 (GDF-9), bone morphogenetic protein 15 (BMP15), kit ligand (KL), hepatocyte growth factor (HGF), and keratinocyte growth factor (KGF); biochemical evaluation of ovarian lipid peroxidation, superoxide dismutase (SOD) and catalase activity; analysis of mitochondrial membrane potential by JC1 staining; detection of granulosa cell apoptosis by TUNEL assay, Hoechst staining and annexin V binding; and Western blot analysis of pro- and anti-apoptotic signaling molecules including p53, bax, bcl2, caspase 3 and cytochrome c.
The follicle-deficient ovary of the sham-operated group demonstrated highly triggered mitochondrial pathways of granulosa cell apoptosis and accelerated follicular atresia. The follicle-deficient ovary of the ovary-transplanted group, by contrast, exhibited stimulated follicle growth with increased expression of GDF-9, BMP15, KL, HGF, KGF, and bcl2 and downregulated expression of p53, bax, caspase 3 and cytochrome c. Both the host and transplanted ovaries also had significantly lower rates of lipid peroxidation with increased SOD and catalase activity. The present results suggest that the balance between the pro-survival and pro-apoptotic factors involved in maintaining optimum intra-follicular communication between germ cell and somatic cells [2-4] is, perhaps, under the upstream regulation of an as-yet unidentified ovarian milieu that is maintained by inter-follicular communications. The declining follicular reserve is possibly the immediate thrust that increases the rate of follicle depletion when the follicle reserve wanes below a certain threshold size.
Labels: MiParea: mt-Membrane, Developmental biology
Stress:Cell death Organism: Rat Tissue;cell: Genital Preparation: Intact Organ"Intact Organ" is not in the list (Intact organism, Intact organ, Permeabilized cells, Permeabilized tissue, Homogenate, Isolated mitochondria, SMP, Chloroplasts, Enzyme, Oxidase;biochemical oxidation, ...) of allowed values for the "Preparation" property.
Regulation: Cyt c, mt-Membrane potential
MiP2014
Affiliation
Cell Biol Physiol Div, CSIR-Indian Inst Chem Biol, Kolkata, India. - [email protected]
Figures
References
- Hale GE, Robertson DM, Burger HG (2013) The perimenopausal woman: Endocrinology and management. J Steroid Biochem Mol Biol doi: 10.1016/j.jsbmb.2013.08.015.
- Gilchrist RB, Ritter LJ, Armstrong DT (2004) Oocyte somatic cell interactions during follicle development in mammals. Anim Reprod Sci 82: 431-46.
- Mazerbourg S, Hseuh AJ (2003) Growth differentiation factor-9 signaling in the ovary. Mol Cell Endocrinol 202: 31-6.
- Vitt UA, Mazerbourg S, Klein C, Hseuh AJ (2002) Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod 67: 473-80.