|
|
| (14 intermediate revisions by 4 users not shown) |
| Line 1: |
Line 1: |
| {{Template:OROBOROS header page name}} | | {{OROBOROS_header_page_name}} |
|
| |
|
| <big><big>'''NextGen-O2k - Next Generation instrument for in-depth mitochondrial status analyses'''</big></big>
| | == Horizon2020 SME Instrument == |
| | [[File:Horizon2020 logo jpg.jpg|280px|Horizon2020 logo jpg.jpg]] |
| | ::::* NextGen-O2k has successfully reached and accomplished Phase 1 of the two-step Horizon2020 SME funding instrument. |
| | ::::* '''Project duration''': 2018-02-01 to 2018-05-31. |
| | ::::* Funding: '''€ 50,000'''. |
|
| |
|
| [[File:Horizon2020 logo jpg.jpg|right|170px|link=https://cordis.europa.eu/project/rcn/213661_en.html]]
| |
| [[File:EU-logo.jpg|right|170px|link=https://ec.europa.eu/commission/index_en]]
| |
| __TOC__
| |
|
| |
|
|
| |
|
| | == Project Output == |
| | The aim of the Phase 1 project was to generate a feasibility study for the development and market launch of the NextGen-O2k and the Q2k. This study consists of a technical plan, a commercial plan and a financial plan and considers technical risks, competition, market conditions and IP rights for a go or no-go decision for the NextGen project: |
|
| |
|
| ==Excellence==
| |
|
| |
|
| | The analysis reveals that we have a strong project from all angles: |
| | ::::* Technically: The project is technically feasible, and we have the required expertise, own personnel and will team up with experienced subcontractors to develop it. In addition, thanks to the expertise and knowledge gained from the development and commercialization of previous products of the company, like the O2k- FluoroRespirometer and its add-on modules we have already defined the needed steps to optimize our new device together with a thorough risk analysis and mitigation/contingency plan. |
| | ::::* Commercially: There is a clear unmet need within our target market, as there are currently no research instruments for mitochondrial investigation that provide an in-depth view of the functional mitochondrial state. Our analysis demonstrates that our target markets will show a constant growth driven by current research trends and the new discoveries of the implication of mitochondria in a wide range of diseases (diabetes, cancer, obesity or neurodegenerative diseases). The Global life science instrumentation market in basic research, healthcare and medical applications is likely to reach $75.24 Billion by 2022, growing at a CAGR of 6.7% from 2017 to 2022. This promising scenario represents a unique opportunity for us. |
| | ::::* Financially: Our financial analysis has demonstrated the economic feasibility of our project, with precisely calculated production costs, which are realistic and in-line with those defined by the actual market situation. Our forecast shows that our business can have a relevant economic impact, enabling us to achieve €46.7 million revenues and €18 million of cumulative net income by 2025, with an ROI of 3.5. |
|
| |
|
| ===Objective===
| | <big>GO DECISION:</big> Based on all the above-mentioned reasons, we have decided to go ahead with our presented project plan and the preparation of the proposal for '''Horizon2020 SME Instrument Phase 2'''. |
| :::: With this new project, [[OROBOROS INSTRUMENTS|Oroboros]] is taking one step ahead in the development of an innovative instrument, the NextGen-O2k, that will allow a more exhaustive and complete analysis of mitochondrial physiological fitness. This bioenergetic snapshot will represent a new standard for the detection of mitochondrial dysfunction in mitochondrial related diseases including hereditary metabolic diseases, different types of cancer, neurodegenerative diseases, cardiovascular diseases, and age-related muscular and cognitive decline. | |
|
| |
|
| :::: As of today, there are several methods available to measure these parameters, such as polarography and fluorometry (for oxygen flux determination) or imaging and fluorometry (to monitor mitochondrial membrane potential in isolated mitochondria or in intact cells). However, most equipments based on these methods are restricted to measuring just one of the two aspects of mitochondrial function (either oxygen flux or membrane potential). Moreover, they present additional drawbacks, such as the lack of precision (due to oxygen leakage which results in poor reproducibility and low sensitivity), low cost-effectiveness (with fragile equipment and high running costs), or limited/poor resolution (due to poor detection limits). | | » Find out more about our H2020 SME phase 2 project: [[NextGen-O2k | NextGen-O2k - The revolutionary all-in-one instrument to conquer mitochondrial disease]] |
| :::: Even though there is an additional and highly versatile indicator of mitochondrial function - the oxidation-reduction (redox) state of the coenzyme Q (Ubiquinone) that is regulated throughout ATP production, directly related to the electron transfer system (ETS) in the mitochondria - none of the currently available instruments is capable of measuring this parameter. Hence, there is a demand for a more advanced integrated system that can fulfil such tasks to reveal mitochondrial fitness.
| |
|
| |
|
| :::: Oroboros NextGen-O2k has been specifically developed to meet this demand. With a core-system based on O2k technology, the NextGen-O2k merges four different add-on modules (a novel Q-redox sensor, autofluorometry, spectrofluorometry, and spectrophotometry) in a single instrument, to measure different biochemical events in the mitochondria, such as oxygen flux, and several redox events of the electron transfer system (ETS). Thus, the NextGen-O2k combines these new features with the high oxygen sensitivity (5 nM), exceptional resolution (wide range for oxygen detection 0-1,000 μM) and the robustness (>20 years lifespan) of Oroboros®’ oxygraphs.3 Moreover, it counts with a uniquely designed software (DatLab), developed to be accessible and easily operated by the users, so that it can perform graphics analysis and statistics from the obtained data. All this together results in a unique, all-in-one cost-competitive equipment (single equipment for different measurements with reduced running costs) with enhanced characteristics (e.g. low oxygen leakage) which allows manageable in-depth studies of mitochondrial dysfunctions, providing a bioenergetic snapshot of cells and mitochondria.
| |
|
| |
|
| :::: Before finalizing the optimization of the NextGen-O2k for its commercialization and to guarantee the success of the project, a feasibility study with the following objectives is required
| |
|
| |
|
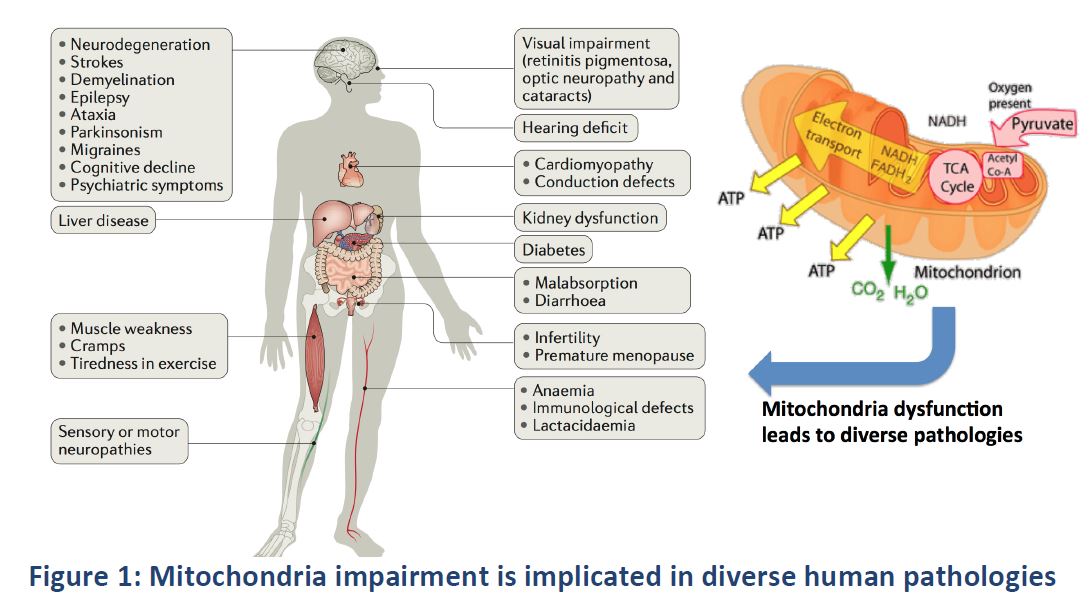
| :::: '''Technical''' | | [[File:Fig1_Feasibility.JPG]] |
| :::::* To assess the best procedure to implement the technical features in the NextGen-O2k (e.g. Q-redox sensor) and to prepare a project execution plan for Phase 2.
| |
| :::::* To define the industrialization scheme to manufacture the NextGen-O2k and manage its distribution.
| |
| :::::* To evaluate the potential of the NextGen-O2k as a medical device.
| |
|
| |
|
| :::: '''Commercial'''
| | Figure taken from the deliverable "Feasibility Study" in the H2020 SME Instrument Project "NEXTGEN-O2K" under grant agreement number 807889 |
| :::::* A market analysis to identify new costumers and regions to reach.
| |
| :::::* To create a business plan to commercialize the NextGen-O2k instrument together with the software.
| |
| :::::* Assessment of extending the distribution capacities.
| |
| | |
| :::: '''Financial'''
| |
| :::::* To carry out a financial study: in-depth cost/benefit analysis on the NextGen-O2k.
| |
| :::::* To update the forecast estimates for a 5-year financial projection and company’s profit.
| |
| :::::* To ensure the forecast production needs are matching with the resources.
| |
| | |
| ===Concept===
| |
| | |
| :::: The NextGen-O2k’s core system is based on the O2k technology, equipped with an advanced O2 sensor (Clark sensor) for High-Resolution Respirometry for the determination of oxygen flux and concentration to perform mitochondria fitness analysis (Fig. 2). The NextGen-O2k includes four additional main modules (Fig. 2), of which three are new (modules 1-3), and one is an extension of O2k technology (module 4):
| |
| :::: 1. An innovative Q-redox sensor, which allows detecting changes in the mitochondrial ETS based on measuring the redox status of the coenzyme Q (Q) pool. This technology was patented by a research lab in 19884 but has never been commercially exploited.
| |
| :::: 2. An autofluorometry tool, which measures oxidation-reduction of NADH, an essential redox molecule of the ETS.
| |
| :::: 3. A spectrophotometry module that allows to detect specific molecules (cytochrome aa3,b,c) in the ETS based on their optical signature to analyse the redox changes in the mitochondria.
| |
| :::: 4. A spectrofluorometry module. In contrast to the previously mentioned modules, which have been specifically developed for the NextGen-O2k, this module is an extension of the module currently available as an add-on for the O2k. In the NextGen-O2k this module (in which the green and blue LEDs with fixed excitation wavelengths are replaced by spectrofluorometers) is integrated directly in the measuring chamber and allows for an accurate detection of oxidative stress through the detection of reactive oxygen species (ROS), membrane potential, ATP and Ca2+ uptake. Spectrofluorometry will extend the range of wavelengths required for application of various fluorophores and allow for ratiometric analyses.
| |
| :::: With the integration of all these modules, a redox fingerprint reflecting mitochondrial core metabolism is finally achieved, providing the user with innovative solutions for in-depth studies of mitochondrial fitness. This hardware in the NextGen-O2k is complemented by the unique and optimized DatLab software, designed for data acquisition and analysis. This software allows several optimizations, such as background correction, it has protocols incorporated to facilitate data acquisition and it allows the analysis and preparation of the graphics of the acquired data.
| |
| | |
| | |
| ==Implementation==
| |
| | |
| === Work plan - work package and deliverable ===
| |
| :::: Description of work
| |
| :::: '''Task 1. Technical feasibility (Months 1-3)''' - ''Task leader: Tímea Komlódi''
| |
| ::::* 1.1. Define the technical steps to optimize Q-redox sensor, electronics and software for NextGen-O2k to complete its final integration.
| |
| ::::* 1.2. Evaluate the potential of the NextGen-O2k system as a medical device.
| |
| ::::* 1.3. Prepare a Project Execution Plan including the timeline and steps foreseen to reach the objectives, the risks and corresponding mitigation measures during Phase 2.
| |
| | |
| :::: '''Task 2. Commercial Feasibility (Months 1-3)''' - ''Task leader: Erich Gnaiger''
| |
| ::::* 2.1. Carry out a comprehensive analysis of the market, assessing the unmet needs in the mitochondrial field and market opportunities worldwide.
| |
| ::::* 2.2. Validate the exploitation strategy, assessing income sources and distribution plans.
| |
| ::::* 2.3. Update the dissemination plan and continue surveying the opinion leaders of the field.
| |
| | |
| :::: '''Task 3. Financial Feasibility (Months 2-4)''' - ''Task leader: Florian Pranger''
| |
| ::::* 3.1. Conduct a full cost/benefit analysis on the NextGen-O2k project, including a projection of total funding needed in Phase 2 to complete the project.
| |
| ::::* 3.2. Review and update the financial projections for the first 5 years of commercialization. Deliverable: Feasibility study report and business plan (after month 4):
| |
| | |
| ::::The findings regarding technical, commercial and financial aspects of the project will be summarized in a report. An undated business plan will be included, with the description of the required activities for the development and commercialization of the NextGen-O2k.
| |
| | |
| === Funding===
| |
| | |
| :::: Total cost: EUR 71.429
| |
| :::: Maximum EU contribution: EUR 50.000
| |
| | |
| This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 807889.
| |
| | |
| | |
| ==Coordinator==
| |
| | |
| ::::* Project coordinator: [[Pranger F|Florian Pranger]]
| |
| ::::* Business manager: [[Gnaiger E|Erich Gnaiger]]
| |
| ::::* Technical manager: [[Komlodi T|Timea Komlodi]]
| |
| ::::: Oroboros Instruments GmbH
| |
| ::::: Schoepfstraße 18
| |
| ::::: 6020 Innsbruck
| |
| ::::: Austria
| |
| | |
| | |
| :::: Official announcement of the H2020 project NextGen-O2k [https://cordis.europa.eu/project/rcn/213661_en.html https://cordis.europa.eu/project/rcn/213661_en.html]
| |