Difference between revisions of "NextGen-O2k H2020 SME Phase 1"
Laner Verena (talk | contribs) |
|||
| (9 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{OROBOROS_header_page_name}} | {{OROBOROS_header_page_name}} | ||
== | == Horizon2020 SME Instrument == | ||
[[File: | [[File:Horizon2020 logo jpg.jpg|280px|Horizon2020 logo jpg.jpg]] | ||
::::* | ::::* NextGen-O2k has successfully reached and accomplished Phase 1 of the two-step Horizon2020 SME funding instrument. | ||
::::* '''Project duration''': | ::::* '''Project duration''': 2018-02-01 to 2018-05-31. | ||
::::* Funding: '''€ 50,000'''. | |||
::::* Funding: '''€ | |||
== Project Output == | |||
The aim of the Phase 1 project was to generate a feasibility study for the development and market launch of the NextGen-O2k and the Q2k. This study consists of a technical plan, a commercial plan and a financial plan and considers technical risks, competition, market conditions and IP rights for a go or no-go decision for the NextGen project: | |||
The analysis reveals that we have a strong project from all angles: | |||
::::* Technically: The project is technically feasible, and we have the required expertise, own personnel and will team up with experienced subcontractors to develop it. In addition, thanks to the expertise and knowledge gained from the development and commercialization of previous products of the company, like the O2k- FluoroRespirometer and its add-on modules we have already defined the needed steps to optimize our new device together with a thorough risk analysis and mitigation/contingency plan. | |||
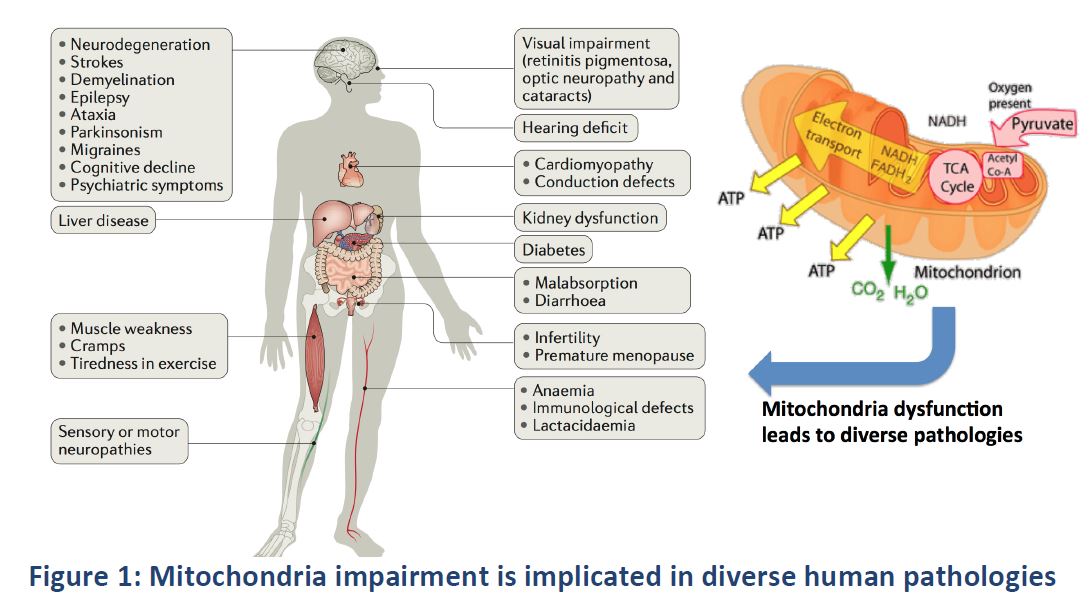
::::* Commercially: There is a clear unmet need within our target market, as there are currently no research instruments for mitochondrial investigation that provide an in-depth view of the functional mitochondrial state. Our analysis demonstrates that our target markets will show a constant growth driven by current research trends and the new discoveries of the implication of mitochondria in a wide range of diseases (diabetes, cancer, obesity or neurodegenerative diseases). The Global life science instrumentation market in basic research, healthcare and medical applications is likely to reach $75.24 Billion by 2022, growing at a CAGR of 6.7% from 2017 to 2022. This promising scenario represents a unique opportunity for us. | |||
::::* Financially: Our financial analysis has demonstrated the economic feasibility of our project, with precisely calculated production costs, which are realistic and in-line with those defined by the actual market situation. Our forecast shows that our business can have a relevant economic impact, enabling us to achieve €46.7 million revenues and €18 million of cumulative net income by 2025, with an ROI of 3.5. | |||
<big>GO DECISION:</big> Based on all the above-mentioned reasons, we have decided to go ahead with our presented project plan and the preparation of the proposal for '''Horizon2020 SME Instrument Phase 2'''. | |||
» Find out more about our H2020 SME phase 2 project: [[NextGen-O2k | NextGen-O2k - The revolutionary all-in-one instrument to conquer mitochondrial disease]] | |||
[[File:Fig1_Feasibility.JPG]] | |||
File: | |||
Figure taken from the deliverable "Feasibility Study" in the H2020 SME Instrument Project "NEXTGEN-O2K" under grant agreement number 807889 | |||
Latest revision as of 09:32, 4 April 2022
NextGen-O2k H2020 SME Phase 1
Horizon2020 SME Instrument
- NextGen-O2k has successfully reached and accomplished Phase 1 of the two-step Horizon2020 SME funding instrument.
- Project duration: 2018-02-01 to 2018-05-31.
- Funding: € 50,000.
Project Output
The aim of the Phase 1 project was to generate a feasibility study for the development and market launch of the NextGen-O2k and the Q2k. This study consists of a technical plan, a commercial plan and a financial plan and considers technical risks, competition, market conditions and IP rights for a go or no-go decision for the NextGen project:
The analysis reveals that we have a strong project from all angles:
- Technically: The project is technically feasible, and we have the required expertise, own personnel and will team up with experienced subcontractors to develop it. In addition, thanks to the expertise and knowledge gained from the development and commercialization of previous products of the company, like the O2k- FluoroRespirometer and its add-on modules we have already defined the needed steps to optimize our new device together with a thorough risk analysis and mitigation/contingency plan.
- Commercially: There is a clear unmet need within our target market, as there are currently no research instruments for mitochondrial investigation that provide an in-depth view of the functional mitochondrial state. Our analysis demonstrates that our target markets will show a constant growth driven by current research trends and the new discoveries of the implication of mitochondria in a wide range of diseases (diabetes, cancer, obesity or neurodegenerative diseases). The Global life science instrumentation market in basic research, healthcare and medical applications is likely to reach $75.24 Billion by 2022, growing at a CAGR of 6.7% from 2017 to 2022. This promising scenario represents a unique opportunity for us.
- Financially: Our financial analysis has demonstrated the economic feasibility of our project, with precisely calculated production costs, which are realistic and in-line with those defined by the actual market situation. Our forecast shows that our business can have a relevant economic impact, enabling us to achieve €46.7 million revenues and €18 million of cumulative net income by 2025, with an ROI of 3.5.
GO DECISION: Based on all the above-mentioned reasons, we have decided to go ahead with our presented project plan and the preparation of the proposal for Horizon2020 SME Instrument Phase 2.
» Find out more about our H2020 SME phase 2 project: NextGen-O2k - The revolutionary all-in-one instrument to conquer mitochondrial disease
Figure taken from the deliverable "Feasibility Study" in the H2020 SME Instrument Project "NEXTGEN-O2K" under grant agreement number 807889