Lai 2014 Abstract1 MiP2014
| Improved isolation of high quality subsarcolemmal and interfibrillar mitochondria from skeletal muscle. |
Link:
Mitochondr Physiol Network 19.13 - MiP2014
Lai N, Kummitha C, Rosca MG, Fujioka H, Hoppel CL (2014)
Event: MiP2014
The subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria in cardiac and skeletal muscle exhibit distinct biochemical and structural properties affecting energy metabolism in health and disease states. The method of isotating mitochondria affects the quality and quantity of the SSM and IFM [4] separated by subcellular fractionation techniques. Previous rat skeletal muscle studies reported lower yield and respiratory acceptor control ratios (RCR) isolated in SSM and IFM [1,2] than isolated in hearts [3]. In these animal studies the functional and structural properties of the mitochondrial subpopulations were not comprehensively investigated. A more recent dog skeletal muscle study [5] used a new isolation protocol for SSM and IFM in which RCR were higher than those obtained in rat studies; the mitochondrial yield was slightly increased only for the IFM population in comparison to those obtained in the rat studies.
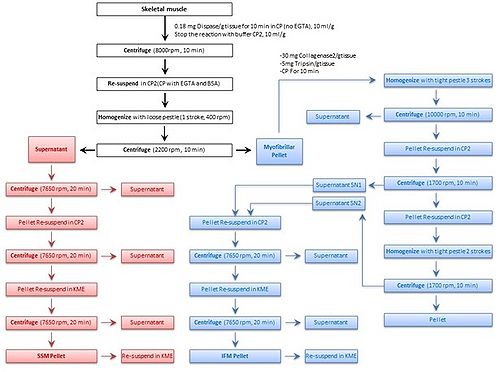
We modified our protocol, based on subcellular fractionation, [5] to improve the isolation of SSM and IFM from rat skeletal muscle. Oxidative phosphorylation, enzymatic, and morphological assays were used to relate functional and structural properties of mitochondria. The major step of the isolation protocol includes skeletal muscle mincing, homogenization, enzymatic treatment and differential centrifugation (Fig. 1). Neutral protease was used to disperse the myofibrils and facilitate the release of SSM during homogenization. The myofibrillar pellet was treated with a combination of trypsin and collgenase type II to extract the IFM population during homogenization.
The yields of SSM and IFM (3.5±0.5; 8.6±1.5 mg∙g-1) from rat skeletal muscle were higher than those previously obtained with rats [1,2] and dog skeletal muscle [5]. The respirometric assay with glutamate as substrate for SSM and IFM showed higher OXPHOS capacity (SSM 307±17; IFM 362±29 nAO∙min-1∙mg-1) and RCR (SSM 19.6±4.2; IFM 18.8±4) than those previously reported in rat skeletal muscle [1,2]. Citrate synthase and succinate dehydrogenase activities were measured to quantify the mitochondrial distribution in the subcellular fractions. Integrated mitochondrial function, measured as oxidative phosphorylation, was used with different substrates to probe oxidation and phosphorylation systems. The activity of respiratory enzyme complexes was measured to quantify the biochemical capacity of ET-pathway components [5]. Electron microscopy images of the subpopulations of mitochondrial confirmed that the procedure preserved the structure SSM and IFM.
The improved method allowed isolation of high quality subpopulations of skeletal muscle mitochondria, comparable to those from hearts [3]. This is a valuable approach to study the relationship between function and structure of skeletal muscle mitochondria in disease conditions.
• O2k-Network Lab: US OH Cleveland Hoppel CL, US OH Cleveland Lai N, US VA Norfolk Lai N
Labels: MiParea: Respiration
Organism: Rat, Dog
Tissue;cell: Heart, Skeletal muscle, Fibroblast
Preparation: Isolated mitochondria
Enzyme: Complex II;succinate dehydrogenase
Regulation: Coupling efficiency;uncoupling
Coupling state: LEAK, OXPHOS
Pathway: N
Event: C2, Oral MiP2014
Affiliation
1-Dep Biomed Engineering; 2-Dep Pediatrics; 3-Dep Pharmacology; 4-Center Mitochondrial Disease; 5-Dep Medicine, School Medicine, Case Western Reserve Univ Cleveland, Ohio, USA. – [email protected]
Figure 1
Figure 1. Oxygen consumption rates in permeabilized soleus and white gastrocnemius muscles of W and GK rats (*P<0.05).
References
- Cogswell AM, Stevens RJ, Hood DA (1993) Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol 264: C383-9.
- Krieger DA, Tate CA, Millin-Wood J, Booth FW (1980) Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol 48: 23–8.
- Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–9.
- Picard M, White K, Turnbull DM (2013) Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three dimensional electron microscopy study. J Appl Physiol 114: 161–71.
- Rosca MG, Okere IA, Sharma N, Stanley WC, Recchia FA, Hoppel CL (2009) Altered expression of the adenine nucleotide translocase isoforms and decreased ATP synthase activity in skeletal muscle mitochondria in heart failure. J Mol Cell Cardiol 46: 927–35.