| Cardoso LHD, Doerrier C, Gnaiger E (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. https://doi.org/10.26124/bec:2021-0001 |
» Bioenerg Commun 2021.1. ![]() published online 2021-06-30
published online 2021-06-30
Cardoso Luiza HD, Doerrier Carolina, Gnaiger Erich (2021) Bioenerg Commun
Abstract: ![]() doi:10.26124/bec:2021-0001
doi:10.26124/bec:2021-0001
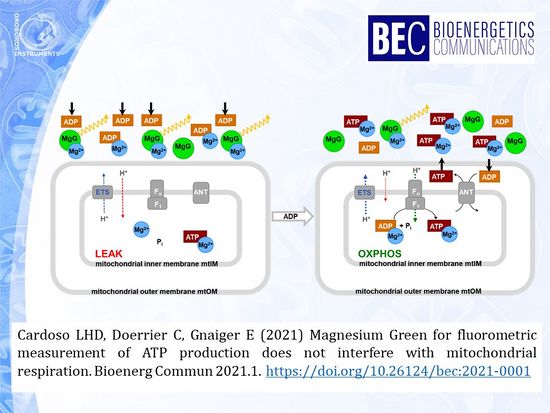
For the advanced study of mitochondrial function, high-resolution respirometry is extended by fluorometric measurement of ATP production using the fluorophore Magnesium Green™ (MgG). A common problem with several fluorescent dyes is the inhibition of mitochondrial respiration. In the present study, a coupling control protocol was applied in combination with MgG to measure ATP production simultaneously with respiration for calculation of P»/O2 ratios. MgG at 1.1 µM did not affect respiration through the NADH-linked and succinate-linked pathways. Respiration was not inhibited in any of the coupling control states, hence coupling control efficiencies were not affected by MgG.
• Keywords: ATP, ATP production, high-resolution respirometry, Magnesium Green, mitochondria, oxidative phosphorylation, fluorometry, FluoRespirometry
• Bioblast editor: Gnaiger E
• O2k-Network Lab: AT Innsbruck Oroboros
ORCID: ![]() Cardoso Luiza HD,
Cardoso Luiza HD, ![]() Doerrier Carolina,
Doerrier Carolina, ![]() Gnaiger Erich
Gnaiger Erich
Data availability
- Original files are available Open Access at Zenodo repository: 10.5281/zenodo.4916141
Support
- Supported by project NextGen-O2k which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 859770. An initiative of the MitoEAGLE Task Group of the Mitochondrial Physiology Society.
Keywords
- Bioblast links: ATP production - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Phosphorylation pathway
- Phosphorylation pathway substrates
- » ADP
- » ATP
- » Inorganic phosphate
- Phosphorylation pathway substrates
- Phosphorylation pathway inhibitors
Coupling control
Respiratory complexes and coupling
ATP production measurement
References (Open Access versus Paywall)
- References - Open Access
| Link | Reference | Year | View |
|---|---|---|---|
| Budinger 1998 J Biol Chem | Budinger GRS, Duranteau J, Chandel NS, Schumacker PT (1998) Hibernation during hypoxia in cardiomyocytes. Role of mitochondria as the O2 sensor. J Biol Chem 273(6):3320-6. | 1998 | PMID:9452449 Open Access |
| Chance 1955 J Biol Chem-I | Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383-93. | 1955 | PMID: 13271402 Open Access |
| Chinopoulos 2014 Methods Enzymol | Chinopoulos C, Kiss G, Kawamata H, Starkov AA (2014) Measurement of ADP-ATP exchange in relation to mitochondrial transmembrane potential and oxygen consumption. Methods Enzymol 542:333-48. doi:10.1016/B978-0-12-416618-9.00017-0 | 2014 | PMID: 24862274 Open Access» |
| Chinopoulos 2009 | Chinopoulos C, Vajda S, Csanady L, Mandi M, Mathe K, Adam-Vizi V (2009) A Novel Kinetic Assay of Mitochondrial ATP-ADP Exchange Rate Mediated by the ANT. Biophys J 96, 2490-504. doi:10.1016/j.bpj.2008.12.3915 | 2009 | PMID: 24391134 Open Access |
| Devaux 2019 Front Physiol | Devaux JBL, Hedges CP, Birch N, Herbert N, Renshaw GMC, Hickey AJR (2019) Acidosis maintains the function of brain mitochondria in hypoxia-tolerant triplefin fish: a strategy to survive acute hypoxic exposure? Front Physiol 9:1941. | 2019 | PMID:30713504 Open Access |
| Doerrier 2018 Methods Mol Biol | Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782:31-70. https://doi.org/10.1007/978-1-4939-7831-1_3 | 2018 | PMID: 29850993 » |
| Fink 2017 Am J Physiol Cell Physiol | Fink BD, Bai F, Yu L, Sivitz WI (2017) Regulation of ATP production: dependence on calcium concentration and respiratory state. Am J Physiol Cell Physiol 313:C146-53. | 2017 | PMID: 28515085 Open Access » |
| Gnaiger 2001 Respir Physiol | Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. https://doi.org/10.1016/S0034-5687(01)00307-3 | 2001 | Respir Physiol 128:277-97. PMID: 11718759 |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 | |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 | Bioenerg Commun 2020.1. |
| Gnaiger 2000 Life in the Cold | Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R (2000) Mitochondria in the cold. In: Life in the Cold (Heldmaier G, Klingenspor M, eds) Springer, Berlin, Heidelberg:431-42. https://doi.org/10.1007/978-3-662-04162-8_45 | 2000 | |
| Gnaiger 2000 Proc Natl Acad Sci U S A | Gnaiger E, Méndez G, Hand SC (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A 97:11080-5. https://doi.org/10.1073/pnas.97.20.11080 | 2000 | PMID: 11005877 Open Access |
| Gnaiger 1994 BTK-207 | Gnaiger E, Wyss M (1994) Chemical forces in the cell: Calculation for the ATP system. In: What is Controlling Life? (Gnaiger E, Gellerich FN, Wyss M, eds) Modern Trends in BioThermoKinetics 3. Innsbruck Univ Press:207-12. | 1994 | |
| Iftikar 2013 PLoS One | Iftikar FI, Hickey AJ (2013) Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS One 8:e64120. | 2013 | PMID: 23724026 Open Access |
| Krumschnabel 2014 Methods Enzymol | Krumschnabel G, Eigentler A, Fasching M, Gnaiger E (2014) Use of safranin for the assessment of mitochondrial membrane potential by high-resolution respirometry and fluorometry. Methods Enzymol 542:163-81. https://doi.org/10.1016/B978-0-12-416618-9.00009-1 | 2014 | PMID: 24862266 » |
| Lark 2016 Am J Physiol Cell Physiol | Lark DS, Torres MJ, Lin CT, Ryan TE, Anderson EJ, Neufer PD (2016) Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol 311:C239-45. | 2016 | PMID: 27335172 Open Access |
| Lemieux 2017 Sci Rep | Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. doi:10.1038/s41598-017-02789-8 | 2017 | PMID: 28588260 Sci Rep Open Access |
| Leyssens 1996 J Physiol | Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR (1996) The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol 496:111-28. | 1996 | Open Access |
| Lowry 1951 J Biol Chem | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-75. | 1951 | PMID:14907713 Open Access |
| Makrecka-Kuka 2015 Biomolecules | Makrecka-Kuka M, Krumschnabel G, Gnaiger E (2015) High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. https://doi.org/10.3390/biom5031319 | 2015 | Biomolecules 5:1319-38. PMID: 26131977 Open Access |
| Masson 2017 Sci Rep | Masson SWC, Hedges CP, Devaux JBL, James CS, Hickey AJR (2017) Mitochondrial glycerol 3-phosphate facilitates bumblebee pre-flight thermogenesis. Sci Rep 7:13107. | 2017 | PMID: 29026172 Open Access |
| Napa 2017 Int J Dent | Napa K, Baeder AC, Witt JE, Rayburn ST, Miller MG, Dallon BW, Gibbs JL, Wilcox SH, Winden DR, Smith JH, Reynolds PR, Bikman BT (2017) LPS from P. gingivalis negatively alters gingival cell mitochondrial bioenergetics. Int J Dent 2017:2697210. | 2017 | PMID: 28592970 Open Access |
| Pham 2014 Am J Physiol | Pham T, Loiselle D, Power A, Hickey AJ (2014) Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am J Physiol 307:C499–507. | 2014 | PMID: 24920675 Open Access |
| Power 2014 Physiol Rep | Power A, Pearson N, Pham T, Cheung C, Phillips A, Hickey A (2014) Uncoupling of oxidative phosphorylation and ATP synthase reversal within the hyperthermic heart. Physiol Rep pii:e12138. | 2014 | PMID: 25263202 Open Access |
| Salin 2019 Proc Biol Sci | Salin K, Villasevil EM, Anderson GJ, Lamarre SG, Melanson CA, McCarthy I, Selman C, Metcalfe NB (2019) Differences in mitochondrial efficiency explain individual variation in growth performance. Proc Biol Sci 286:20191466. | 2019 | PMID: 31431161 Open Access |
| Salin 2018 Integr Comp Biol | Salin K, Villasevil EM, Anderson GJ, Selman C, Chinopoulos C, Metcalfe NB (2018) The RCR and ATP/O indices can give contradictory messages about mitochondrial efficiency. Integr Comp Biol 58:486-94. | 2018 | PMID: 29982616 Open Access |
| Salin 2016 Physiol Rep | Salin K, Villasevil EM, Auer SK, Anderson GJ, Selman C, Metcalfe NB, Chinopoulos C (2016) Simultaneous measurement of mitochondrial respiration and ATP production in tissue homogenates and calculation of effective P/O ratios. Physiol Rep 10.14814/phy2.13007. | 2016 | PMID: 27798358 Open access |
| Scaduto 1999 Biophys J | Scaduto RC Jr, Grotyohann LW (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76:469-77. | 1999 | PMID:9876159 Open Access |
| MiPNet03.02 Chemicals-Media | Selected media and chemicals for respirometry with mitochondrial preparations. | 2016-08-30 | |
| MiPNet20.06 IsolationMouseHeart-mt | Laboratory protocol: isolation of mouse heart mitochondria. | 2021-08-09 |
- References - Paywall
| Link | Reference | Year | View |
|---|---|---|---|
| Goo 2013 Clin Exp Pharmacol Physiol | Goo S, Pham T, Han JC, Nielsen P, Taberner A, Hickey A, Loiselle D (2013) Multiscale measurement of cardiac energetics. Clin Exp Pharmacol Physiol 40:671-81. | 2013 | PMID: 23745944 |
| Horgan 1978 Aust J Biol Sci | Horgan DJ (1978) A spectrophotometric assay of ATP synthesized by sarcoplasmic reticulum. Aust J Biol Sci 31(1):21-4. | 1978 | PMID:98139 |
| Manfredi 2002 Methods | Manfredi G, Yang L, Gajewski CD, Mattiazzi M (2002) Measurements of ATP in mammalian cells. Methods 26:317-26. | 2002 | PMID: 12054922 |
| Menegollo 2019 Methods Mol Biol | Menegollo M, Tessari I, Bubacco L, Szabadkai G (2019) Determination of ATP, ADP, and AMP Levels by Reversed-Phase High-Performance Liquid Chromatography in Cultured Cells. Methods Mol Biol 1925:223-32. | 2019 | PMID:30674030 |
| Morciano 2017 Nat Protoc | Morciano G, Sarti AC, Marchi S, Missiroli S, Falzoni S, Raffaghello L, Pistoia V, Giorgi C, Di Virgilio F, Pinton P (2017) Use of luciferase probes to measure ATP in living cells and animals. Nat Protoc 12(8):1542-62. | 2017 | PMID:28683062 |
| Sausen 2019 Methods Mol Biol | Sausen CW, Rogers CM, Bochman ML (2019) Thin-Layer Chromatography and Real-Time Coupled Assays to Measure ATP Hydrolysis. Methods Mol Biol 1999:245-253. | 2019 | PMID:31127581 |
Cited by
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
Preprint
Labels: MiParea: Respiration, Instruments;methods
Organism: Mouse
Tissue;cell: Heart
Preparation: Isolated mitochondria
Regulation: ATP production, Coupling efficiency;uncoupling Coupling state: LEAK, OXPHOS, ET Pathway: N, S, ROX HRR: Oxygraph-2k, O2k-Fluorometer, NextGen-O2k, O2k-Protocol
SUIT-006, MgG, MitoEAGLEPublication, BEC2021.5, MitoFit 2022 NADH, O2k-Demo, ATP